Successfully challenging a drug patent requires strategic legal, regulatory, and technical approaches to overcome brand-name manufacturers’ defenses. Here are the key strategies backed by industry practices and case law:
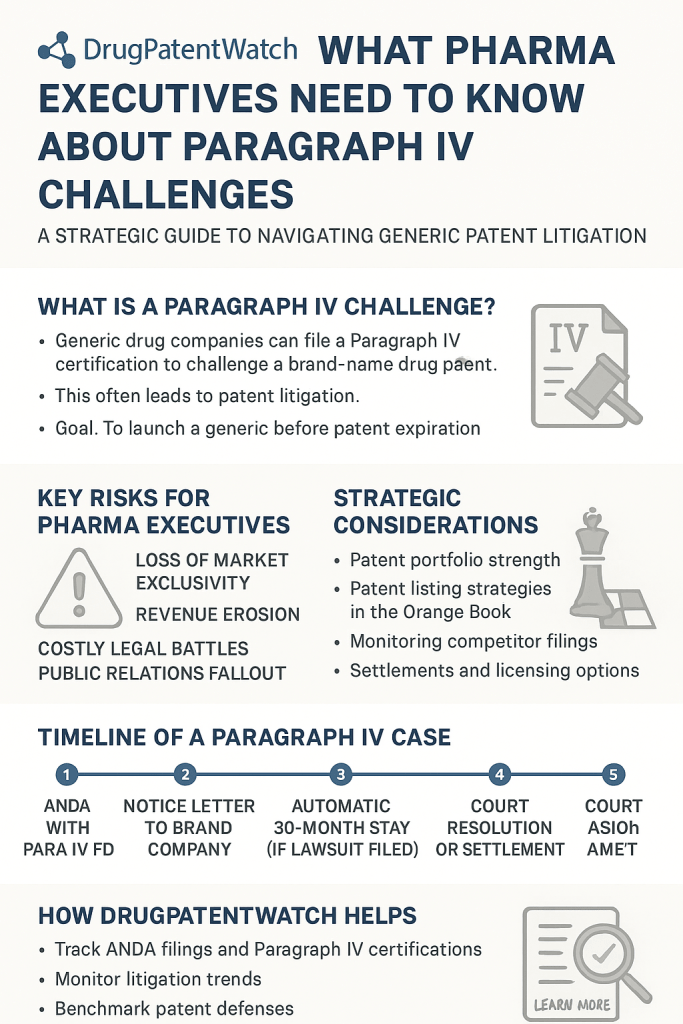
1. File Paragraph IV Certification Early
Generic manufacturers initiate challenges by filing an Abbreviated New Drug Application (ANDA) with a Paragraph IV certification, asserting the patent is invalid, unenforceable, or not infringed. This tactic triggers a 45-day window for the brand-name company to sue, starting the litigation clock.
– Critical advantage: First filers gain 180-day exclusivity if successful[3][16].
– Risk: Triggers a 30-month FDA approval stay if litigation begins, but early challenges shorten delays[6][16].
2. Target Weak Patents Through Prior Art and Validity Challenges
Invalidating patents requires exposing flaws in their novelty, non-obviousness, or utility:
– Prior art analysis: Identify existing publications or patents predating the invention. Drug labels (with timestamps) and scientific literature are potent prior art sources[4][15].
– Prosecution history review: Look for inconsistencies in patent applications (e.g., narrowing claims to gain approval)[2][7].
– Secondary patents: Challenge peripheral patents on formulations or methods of use, which are easier to invalidate than active-ingredient patents[15][16].
– Example: Genentech v. Sandoz saw claims rejected for obviousness, as adjusting drug doses was deemed routine[9].
3. Leverage PTAB Proceedings
The Patent Trial and Appeal Board (PTAB) offers faster, cheaper challenges via Inter Partes Review (IPR):
– Success rate: PTAB invalidates claims in ~60-70% of cases[2][4].
– Best practices: Combine IPR with district court litigation to pressure patent holders[2][9].
– Limitation: Estoppel applies if IPR fails, blocking later use of same arguments in court[2][4].
4. Negotiate Strategic Settlements
Settlements can expedite market entry while avoiding litigation risks:
– Early entry agreements: Generic manufacturers may secure entry dates before patent expiry[2][14].
– Reverse payments: Controversial but legally permissible if pro-competitive (e.g., sharing production capabilities)[14][16].
– Avoid pitfalls: Ensure terms comply with antitrust laws to prevent enforcement actions[14][16].
5. Exploit Regulatory and Legal Loopholes
- Orange Book delisting: Challenge improper listings (e.g., manufacturing patents) to remove 30-month stays[15][17].
- Authorized generics: Collaborate with brand-name firms to launch competing generics during litigation[12][16].
- Product hopping counterstrategies: Block brand attempts to replace drugs with patented variants (e.g., Actavis’ Namenda case)[12].
6. Optimize Litigation Venues and Tactics
- Favorable jurisdictions: File in districts like Delaware, where judges have pharma expertise[2][7].
- Expert witnesses: Use pharmacologists or chemists to dissect patent claims[5][7].
- Parallel global challenges: Coordinate with international teams to invalidate patents in key markets[5][11].
7. Advocate for Policy Reforms
- Expand exclusivity incentives: Prolong 180-day exclusivity in small markets to encourage challenges[6][17].
- Price negotiation laws: Push for Medicare drug price caps to weaken brand pricing power[6][15].
- Patent transparency: Lobby for stricter Orange Book listing criteria to reduce bogus patents[15][17].
Anticipate Brand-Name Counterstrategies
Brands often retaliate by:
– Patent thicketing: Listing dozens of overlapping patents post-challenge (e.g., 0.34 extra patents per Paragraph IV filing)[11].
– Continuation applications: Filing iterative patents to extend protection[3][11].
– Authorized generics pre-launch: Undercut prices before exclusivity periods start[12].
Key Takeaways
Successful challenges combine aggressive litigation, meticulous prior art research, and regulatory savvy. Generic firms must balance early entry rewards against litigation costs, while policymakers can accelerate competition by reforming Hatch-Waxman loopholes. For high-value drugs, collaborations between manufacturers and advocacy groups (e.g., AIDS Access Foundation in Thailand’s didanosine case[13]) amplify pressure on patent holders.
References
- https://www.commonwealthfund.org/publications/journal-article/2017/sep/strategies-delay-market-entry-generic-drugs
- https://www.drugpatentwatch.com/blog/winning-drug-patent-disputes-proven-strategies-for-pharmaceutical-companies/
- https://www.upcounsel.com/how-long-does-a-drug-patent-last
- https://ipwatchdog.com/2019/11/13/curing-drug-label-prior-art-malady-ptab/id=115886/
- https://patentpc.com/blog/patent-litigation-in-the-pharmaceutical-industry-key-considerations
- https://pmc.ncbi.nlm.nih.gov/articles/PMC11867330/
- https://patentpc.com/blog/how-patent-litigation-influences-drug-approvals-and-market-entry/
- https://www.greyb.com/blog/strategy-for-generic-pharma-companies/
- https://www.drugpatentwatch.com/blog/handling-drug-patent-invalidity-claims/
- https://www.journals.uchicago.edu/doi/10.1162/AJHE_a_00066
- https://events.bse.eu/live/files/3828-manuscript02242022anonymizedpdf
- https://pmc.ncbi.nlm.nih.gov/articles/PMC4915805/
- https://www.patentoppositions.org/en/case_studies/500e9b8c7718ea0002000018
- https://scholarship.law.georgetown.edu/facpub/574/
- https://www.healthaffairs.org/do/10.1377/forefront.20200827.532806/
- https://www.ajmc.com/view/a636-article
- https://www.theregreview.org/2024/05/28/challenging-drug-patents-to-lower-prices/