Executive Summary
Technological advancements, particularly in Artificial Intelligence (AI) and Machine Learning (ML), alongside sophisticated manufacturing techniques, are profoundly reshaping the generic drug development lifecycle. These innovations are not merely incremental improvements but represent a fundamental transformation, leading to accelerated timelines, significant cost reductions, enhanced product quality, and the enablement of more complex and personalized formulations. The pharmaceutical sector is poised for substantial value creation from AI, with projections indicating an annual generation of $350 billion to $410 billion by 2025, and the global AI in pharmaceutical market is estimated to reach approximately $16.49 billion by 2034.1
The adoption of these technologies is proving to be a critical strategic imperative for generic companies to maintain economic viability and enhance their competitive standing. Generic manufacturers frequently operate with razor-thin profit margins and face a “downward spiral” due to intense market pressures.2 The substantial cost reductions (up to 40% in drug discovery) and timeline accelerations (up to 70% in development) achievable through AI/ML represent a vital mechanism for these companies to sustain their operations and capture market share, particularly within the evolving landscape of complex generics.1 Without these advancements, the traditional generic business model faces considerable sustainability challenges.
Beyond economic advantages, technology serves as a powerful enabler for broader public health impact and market expansion. Digital technologies facilitate the creation of more intricate generic formulations, including combination products and biosimilars, which directly translate to improved treatment options and patient outcomes, alongside lower prices.7 Generics already contribute significantly to healthcare savings, generating a record $408 billion in 2022 and $2.9 trillion over the past decade in the U.S. healthcare system.8 By enabling the development of complex and higher-value generics, technology expands the addressable market for these products beyond simple small molecules. This directly results in greater patient access to a wider array of affordable, high-quality, and therapeutically advanced medications, thereby reinforcing and expanding the public health mission of the generic pharmaceutical industry.
However, the full realization of these benefits hinges on addressing critical challenges related to regulatory adaptation, data security, and ethical considerations. Strategic investment in technology, robust talent development, and proactive, collaborative efforts between industry and regulatory bodies are essential to navigate these complexities and fully leverage the transformative potential of these innovations.
1. Introduction: The Evolving Landscape of Generic Drug Development
1.1 Importance of Generic Drugs and Their Traditional Lifecycle
Generic drugs are indispensable pillars of global healthcare systems, playing a pivotal role in enhancing medication affordability and accessibility for patients worldwide. In the United States, for instance, generic medicines account for a remarkable 90% of all prescriptions filled, yet they represent a mere 17.5% of the total prescription drug spending. This disparity underscores their immense economic value, contributing to record savings of $408 billion in 2022 alone and an astounding $2.9 trillion over the past decade for the U.S. healthcare system and its patients.8 This financial relief allows healthcare systems to allocate resources to other critical areas, including the financing of innovative new active ingredients.10
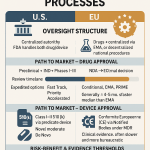
The traditional development process for generic drugs, primarily governed by the Abbreviated New Drug Application (ANDA) pathway in the U.S., is characterized by its inherent complexity and lengthy timelines. Typically spanning 3 to 4 years from initial development to final regulatory approval, this process involves several meticulous stages.11 These stages include pre-application research and development, which involves identifying a suitable brand-name drug for replication, developing a bioequivalent formulation, and conducting preliminary safety and efficacy studies.12 Following this, a comprehensive ANDA must be submitted to the FDA, detailing the drug’s composition, manufacturing processes, quality control measures, results of bioequivalence studies, and proposed labeling.12 The FDA then undertakes a thorough review, which may involve queries and concerns that the generic company must promptly address, often requiring additional data or studies.12 The ultimate goal is final approval and market launch.12 The bedrock of generic drug approval is the rigorous demonstration of bioequivalence, ensuring that the generic version delivers the same amount of active ingredient to the bloodstream at the same rate as the brand-name reference listed drug, thereby guaranteeing equivalent therapeutic effect.12
A fundamental tension exists within the generic pharmaceutical industry: the market demands increasingly lower-cost medications, yet the underlying development and regulatory hurdles for producing these drugs remain substantial. The process, even for generics, is described as “complex” and “long and expensive”.11 This creates a challenging environment where companies must continually innovate to meet pricing pressures while navigating stringent development and approval requirements. This inherent conflict highlights precisely why technological advancements are becoming indispensable. By streamlining processes, enhancing precision, and reducing costs, these innovations enable the generic industry to sustain its critical role in providing affordable healthcare solutions.
Historically, the Hatch-Waxman Act of 1984 served as a landmark regulatory innovation that profoundly reshaped the generic drug industry. By creating the ANDA pathway, it simplified generic approvals based on proof of bioequivalence, effectively bypassing the need for generic manufacturers to repeat costly and time-consuming efficacy and safety research already conducted for the brand-name drug.13 This act fundamentally transformed the landscape, enabling a rapid growth phase for generics, with a projected annual savings of $1 billion in its first year alone.13 The current wave of AI/ML and advanced manufacturing technologies can be viewed as the next evolutionary leap, moving beyond regulatory simplification to deep process and product innovation. Just as Hatch-Waxman provided a foundational framework for generic market entry, modern technologies are now providing the advanced means to overcome new complexities, such as those associated with complex generics, and to push the boundaries of what is possible in generic product development. This historical parallel underscores how regulatory foresight can create fertile ground for subsequent technological advancements, allowing the industry to address emerging challenges and continually expand access to affordable medicines.
1.2 Overview of Key Technological Advances
The pharmaceutical industry is on the cusp of a profound transformation, with Artificial Intelligence (AI) projected to generate between $350 billion and $410 billion annually for the sector by 2025. The global AI in pharmaceutical market is estimated at $1.94 billion in 2025 and is forecasted to reach approximately $16.49 billion by 2034, accelerating at a compound annual growth rate (CAGR) of 27%.1 This substantial market expansion signals a growing reliance on AI to drive innovation and efficiency across the entire pharmaceutical value chain.
Key technological advancements exerting a significant influence on generic drug development include:
- Artificial Intelligence (AI) and Machine Learning (ML): These technologies are being extensively applied across the entire drug development lifecycle. Their applications range from accelerating initial drug discovery and target identification to optimizing drug formulation, predicting bioequivalence, streamlining clinical trial design, and enhancing post-market surveillance.1 Generative AI, a specialized subset of AI, is particularly impactful in accelerating early design efforts and facilitating the creation of novel molecular structures.4
- Advanced Manufacturing Technologies (AMTs): This category encompasses innovations such as Continuous Manufacturing (CM) and 3D printing. These technologies hold immense promise for enhancing product quality, improving manufacturing efficiency, increasing production flexibility, and enabling the development of complex and personalized drug formulations. Over the long term, they also offer the potential for significant cost reductions.2
- Automation and Robotics: These technologies are instrumental in streamlining various production steps within pharmaceutical manufacturing, including liquid handling, precise weighing, accurate dispensing, and meticulous inspection of components and finished drug products. Their integration is designed to minimize human error, reduce manual interventions, and accelerate production cycles, leading to smoother, more efficient, and highly controlled operations.15
- Bioinformatics: This interdisciplinary field leverages computational tools and statistical methods to analyze vast biological datasets. In drug development, bioinformatics expedites the identification of drug targets, enhances the screening and optimization of potential drug candidates, and facilitates the detailed characterization of side effects and the prediction of drug resistance.28
- Advanced Analytics: This broad category includes powerful tools such as AI, ML, and data mining. Advanced analytics accelerates drug discovery, development, manufacturing, and supply chain optimization by enabling the analysis of massive datasets, providing predictive insights, and facilitating real-time monitoring and informed decision-making across the pharmaceutical value chain.17
The true transformative power in generic drug development stems not from the isolated adoption of individual technologies but from their synergistic integration into a comprehensive digital ecosystem. While each technology offers distinct advantages, their combined application yields far greater benefits. For instance, AI algorithms can optimize continuous manufacturing processes 26, and advanced analytics can power predictive maintenance strategies.17 This interconnectedness suggests that generic companies must pursue a holistic digital strategy where the combined impact of these technologies significantly exceeds the sum of their individual parts, leading to more profound improvements in efficiency, quality, and speed.
Furthermore, these advancements signify a crucial paradigm shift in the focus of generic drug development. While many discussions about AI in pharmaceuticals often center on “drug discovery,” traditionally associated with novel, branded drugs 1, the application of AI is explicitly broadening to encompass the “lifecycle management of generic drugs”.16 This includes stages from active pharmaceutical ingredient (API) synthesis to regulatory compliance. This indicates a profound change for the generic sector: the emphasis is shifting from merely replicating an existing drug to optimizing
every single stage of its development, manufacturing, and market entry. This deeper, more comprehensive application of technology is essential for addressing the unique challenges and intense competitive pressures inherent in the generic market, ultimately enabling the more efficient and precise development of high-quality, bioequivalent products.
1.3 Purpose and Structure of the Report
This report aims to provide a comprehensive and expert-level analysis of how technological advancements are fundamentally reshaping the generic drug development landscape. It will meticulously detail the specific applications of Artificial Intelligence (AI) and Machine Learning (ML), advanced manufacturing techniques, automation, and bioinformatics across the entire generic drug development lifecycle. Furthermore, the report will quantify their transformative impacts on key metrics such as cost, time, and quality, while also addressing the critical challenges and strategic considerations necessary for successful adoption. The report concludes with a forward-looking perspective on emerging trends, including personalized generics, and offers actionable conclusions for both industry stakeholders and regulatory bodies to foster continued innovation and ensure broader patient access to affordable, high-quality medicines.
2. Technological Advances Revolutionizing Generic Drug Development Stages
2.1 Artificial Intelligence and Machine Learning (AI/ML)
Artificial Intelligence (AI) is rapidly becoming a dominant force within the pharmaceutical industry, projected to generate between $350 billion and $410 billion annually for the sector by 2025. The global AI in pharmaceutical market, valued at $1.94 billion in 2025, is forecasted to soar to approximately $16.49 billion by 2034, demonstrating a robust compound annual growth rate (CAGR) of 27%.1 This substantial market expansion underscores the increasing reliance on AI to drive innovation and efficiency across the entire pharmaceutical value chain.
2.1.1 Accelerating Drug Discovery and Target Identification
AI and Machine Learning (ML) are revolutionizing the early stages of drug development by significantly accelerating target identification and the screening of drug candidates. These technologies enable the analysis of vast datasets, allowing for the precise identification of potential drug targets with greater accuracy and efficiency. By predicting intricate drug-target interactions, AI helps in pinpointing promising compounds much earlier in the development process.1 This capability inherently increases the likelihood of clinical success by making the entire drug development process not only faster but also considerably smarter.1
Generative AI, a particularly transformative subset of AI, is fundamentally altering drug design. It shifts the paradigm from merely screening existing libraries of molecules to generating entirely new drug molecules from scratch.4 This innovative approach can significantly cut early research and development (R&D) cycles by up to 70%.6 A notable example of this impact is Insilico Medicine, which leveraged Generative AI to advance from novel-target discovery to a preclinical candidate in an astonishing 13-18 months, at a fraction of the traditional cost, approximately $2.6 million.6 Furthermore, AI assists in predicting the complex 3D structures of proteins, such as through technologies like AlphaFold. This capability is crucial for designing more effective drugs and accelerating the discovery of treatments for challenging diseases like Alzheimer’s and cancer.4 Such predictive power allows researchers to concentrate their efforts on the most promising compounds, thereby saving considerable time and resources.4
These advanced capabilities are crucial for bridging the innovation gap, particularly for complex generics. While “drug discovery” is traditionally associated with novel, branded drugs, AI’s ability to predict drug properties and optimize drug candidates is profoundly relevant for generic drug development, especially for “complex generics”.2 These sophisticated tools enable generic companies to more efficiently reverse-engineer intricate formulations, understand subtle drug-excipient interactions, and develop bioequivalent versions that go beyond simple active pharmaceutical ingredient (API) replication to a more sophisticated understanding and re-creation of the entire drug product. This significantly enhances their capability to compete effectively in higher-value generic segments.
Moreover, the advent of AI is democratizing advanced R&D capabilities across the pharmaceutical landscape. Historically, cutting-edge drug discovery tools and high-throughput screening were largely the exclusive domain of large, well-funded innovative pharmaceutical companies. However, AI, particularly generative AI, with its capacity to generate entirely new drug molecules and significantly halve bench-scale synthesis time and costs, is making these advanced R&D capabilities more accessible.4 This accessibility empowers smaller generic manufacturers and biotech startups to participate more effectively in developing complex or differentiated generic products. This fosters greater competition and innovation within the generic sector, which has traditionally focused primarily on the speed and cost of replication.
2.1.2 Optimizing Formulation and Excipient Selection
AI and ML are revolutionizing generic drug formulation by enhancing efficiency, precision, and cost-effectiveness throughout the process.16 These technologies facilitate predictive modeling, comprehensive risk assessment, and the optimization of drug formulation processes, thereby significantly reducing time-to-market and improving scalability.16
AI can meticulously analyze the physicochemical properties of various drug molecules and excipients (inactive ingredients) to optimize formulations for improved drug delivery, stability, and bioavailability.22 The integration of computational optimization with AI dramatically reduces the reliance on conventional, often time-consuming, and resource-intensive trial-and-error approaches that have historically characterized formulation development.19 This represents a fundamental shift from empirical to predictive formulation development. AI/ML transforms formulation from reactive experimentation to a proactive, data-driven design process. This not only yields substantial savings in time and resources but also enables the exploration of a much broader “formulation design space,” leading to the development of more robust, stable, and higher-quality generic products from their initial conception.16
Specific industry examples highlight this transformative impact. Merck, for instance, has developed an innovative AI-based tool capable of predicting potential compatible co-formers for co-crystallization. This process can improve drug solubility, potentially preventing effective active ingredients from being shelved due to formulation challenges.35 Furthermore, machine learning algorithms, such as ExPreSo, have been specifically developed to suggest optimal inactive ingredients (excipients) based on the properties of the protein drug substance and the target product profile. This demonstrates high prediction accuracy and resilience, marking a significant advancement in rational excipient selection.36
The ability of AI to optimize excipient selection and accurately predict properties like solubility and stability is particularly critical for enabling complex generic formulations and patient-centric design.16 These capabilities are essential for the successful development of “complex generics” 7, such as liposomal formulations or nanosuspensions 18, which require intricate understanding and control over their physical and chemical properties. For example, improving a drug’s solubility through AI-guided formulation can facilitate the development of an oral tablet instead of an injection, a dosage form often preferred by patients, thereby improving patient compliance and convenience.35 This demonstrates that AI is not merely accelerating existing processes but actively enabling the creation of generic products with enhanced therapeutic profiles and improved patient compliance, thereby moving generic development towards a more patient-centric and value-added approach.
2.1.3 Enhancing Bioequivalence and Bioavailability Prediction
Bioequivalence (BE) stands as the cornerstone requirement for generic drug approval, serving as the primary assurance that a generic drug delivers the same amount of active ingredient to the bloodstream at the same rate as its brand-name counterpart, thus ensuring equivalent therapeutic effect.12 AI and Machine Learning (ML) are increasingly being leveraged to streamline and enhance the prediction and assessment of bioequivalence, a critical regulatory step in generic drug development.16
Machine learning models, such as an optimized Random Forest model, have demonstrated a high accuracy of 84% in predicting BE risk by utilizing a range of pharmacokinetic and physicochemical characteristics of drugs. Key predictive features include solubility (e.g., dose number, acid dissociation constant), absorption and elimination rates, effective permeability, variability of pharmacokinetic endpoints, and absolute bioavailability.38 These features provide a comprehensive understanding of a drug’s behavior within the body, allowing for more informed predictions.
Advanced generative models, specifically Wasserstein Generative Adversarial Networks (WGANs), show significant promise in creating virtual subjects for BE trials. This innovative approach has the potential to reduce the need for actual human participants, enhance the effective sample sizes, substantially reduce costs, and accelerate trial durations, thereby addressing some of the most resource-intensive aspects of generic development.39
Furthermore, Physiologically Based Pharmacokinetics (PBPK) modeling, often augmented by AI, is instrumental in defining a “BE safe space.” This allows for the identification of newer bioequivalent formulations and provides a deeper mechanistic understanding of drug absorption and critical bioavailability attributes.40 PBPK models can also predict and help circumvent potential drug-drug interactions, contributing to better efficacy and patient safety.40
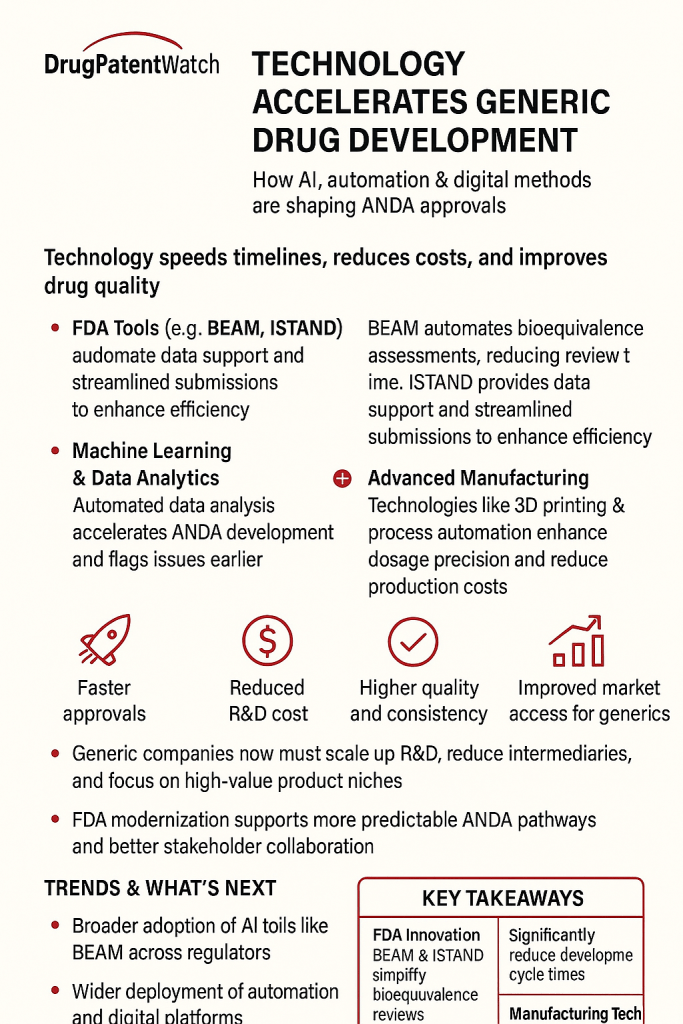
Regulatory bodies are also embracing these technological shifts. The U.S. Food and Drug Administration (FDA) is actively developing tools such as the Bioequivalence Assessment Mate (BEAM), a data and text analysis tool designed to automate labor-intensive tasks and improve the overall efficiency of the BE assessment process. This tool is anticipated to save significant time for reviewers, thereby expediting the approval pathway for generics.3
The application of AI and ML for BE prediction and virtual trials is crucial for de-risking and accelerating what is often the foremost regulatory bottleneck for generics. Bioequivalence studies are typically the most critical and resource-intensive regulatory hurdle for generic drug approval.12 By accurately predicting BE outcomes
in silico or through virtual populations, generic companies can significantly de-risk their development programs, reduce costly late-stage failures, and accelerate the time to Abbreviated New Drug Application (ANDA) submission and approval. This represents a fundamental shift from a purely empirical, post-hoc assessment to a more predictive and efficient approach, which is vital for competitive market entry.
This embrace of technology also highlights a significant regulatory evolution driven by advanced technological capabilities. The FDA’s proactive development of tools like BEAM 3 and its issuance of draft guidance on the use of AI to support regulatory decision-making 41 indicate that regulatory bodies are not merely reacting to technological advancements but are actively integrating them into their assessment processes. This suggests a future where AI-generated data and predictive models might become increasingly acceptable, or even expected, for regulatory submissions. This implies a collaborative evolution where industry’s technological innovations incentivize regulators to adapt and innovate their assessment tools, ultimately leading to a more efficient, data-driven, and potentially faster approval ecosystem for generic drugs.
2.1.4 Streamlining Clinical Trial Design and Patient Recruitment
Artificial Intelligence (AI) plays a pivotal role in optimizing clinical trial design and patient recruitment through sophisticated predictive analytics.15 By analyzing vast datasets, AI significantly increases the likelihood of clinical success by identifying promising drug candidates earlier in the development pipeline.1
AI substantially improves patient selection for clinical trials, leading to more targeted and effective studies. This reduces the likelihood of trial failure due to inappropriate patient populations and can potentially shorten trial durations.4 Tools like TrialGPT automate the process of matching patients to suitable trials by analyzing extensive Electronic Health Records (EHRs). This not only accelerates recruitment but also helps ensure greater diversity in trials and can even predict patient dropouts, thereby preventing costly trial disruptions.1
AI algorithms are capable of identifying specific patient subgroups who are most likely to respond positively to treatments. This capability enables real-time adjustments to trial protocols and allows for the refinement of inclusion criteria. This dynamic approach can reduce overall trial duration by up to 10% without compromising the integrity of the collected data.1 Furthermore, AI facilitates real-time data analysis during trials, continuously processing patient data to identify emerging trends, predict outcomes, and optimize protocols on the fly. This dynamic data analysis helps to optimize the trial, reduce the likelihood of errors, and ultimately save significant resources. Generative AI can even predict the success rate of a trial by analyzing historical data, thereby increasing the probability of success and potentially yielding up to $25 billion in savings in clinical development alone.1
These advancements are crucial for mitigating the high failure rates and costs typically associated with clinical development. Clinical trials represent a major bottleneck in drug development, characterized by substantial costs and significant failure rates.6 AI’s ability to increase the likelihood of clinical success 1 and reduce the probability of trial failure 4 directly addresses this core challenge. By optimizing patient selection and trial design, AI shifts the focus from broad, expensive trials to more precise, efficient studies. While generic drugs typically follow an Abbreviated New Drug Application (ANDA) pathway that often bypasses extensive human clinical trials for efficacy, these AI capabilities are particularly crucial for complex generics and biosimilars that may still require some level of human clinical demonstration. This directly improves their overall success rate and accelerates their market entry.
The application of AI also contributes to the emergence of adaptive and real-time clinical development. The traditional model of clinical trials is often rigid and sequential, with data analysis typically occurring at fixed intervals. AI, by enabling real-time data analysis and dynamic adjustments to trial protocols 1, facilitates a more adaptive and responsive approach to clinical development. This suggests a future where trials are continuously optimized based on incoming data, moving away from fixed, lengthy phases to a continuous learning and refinement process. For generic companies, especially those developing complex products, this adaptive framework could significantly de-risk and accelerate the necessary human studies, making them more efficient and targeted, and ultimately bringing these critical medicines to patients faster.
2.2 Advanced Manufacturing Technologies (AMTs)
The adoption of Advanced Manufacturing Technologies (AMTs) represents one of the most promising avenues for innovation in generic drug production. These technologies are poised to significantly enhance product quality, improve manufacturing efficiency, increase production flexibility, and potentially reduce costs over the long term.2
2.2.1 Continuous Manufacturing (CM)
Continuous Manufacturing (CM) signifies a profound paradigm shift from traditional batch manufacturing processes in pharmaceutical production. Unlike batch processing, which involves discrete production steps with interruptions between them, CM integrates all production stages into a seamless, uninterrupted flow.2 This integration allows for a more streamlined and efficient production line.
The benefits of CM for generic producers are substantial and multifaceted. These include improved quality control through real-time monitoring, which allows for immediate detection and correction of deviations, ensuring consistent product quality.2 CM also leads to a reduced manufacturing footprint, as it requires less physical space compared to traditional batch facilities.2 Production times are significantly faster, potentially reducing manufacturing cycles from weeks to mere days.2 Furthermore, CM offers greater flexibility to respond dynamically to demand fluctuations, enhancing supply chain responsiveness.2 From an economic standpoint, CM can lead to decreased manufacturing expenses through a reduced facility footprint, lower energy consumption, and improved yields.2 It also inherently reduces the risk of product contamination and quality issues due to the closed and continuous nature of the process.26
Despite these compelling advantages, a notable gap in adoption exists within the generic sector. While several branded drugs have successfully received approval using CM, no generic drugs have yet achieved approval via this method.2 This observation highlights a substantial untapped potential for generic companies. Early adopters of CM could establish a significant competitive advantage by achieving superior cost structures and quality profiles compared to those relying on traditional batch processes. This also suggests that the current regulatory landscape or the high upfront investment required for CM infrastructure may act as a barrier to its wider adoption by generic manufacturers.
The role of regulatory frameworks is critical in either enabling or inhibiting the widespread adoption of CM. The U.S. Food and Drug Administration (FDA) has recognized CM as a method that can help prevent drug shortages and enhance product quality.2 However, the limited number of generic approvals via CM, coupled with the acknowledgment that “regulatory frameworks for CM in personalized medicine are still evolving and need to be clarified” 26, indicates that the regulatory environment plays a crucial role. This implies that while the technological benefits of CM are clear, regulatory bodies need to further develop and clarify guidelines, and potentially create incentives, to facilitate widespread adoption of CM by generic manufacturers. Such policy evolution could transform the regulatory landscape into a key driver for industry transformation, ultimately benefiting patients through more reliable and affordable drug supplies.
2.2.2 3D Printing for Customized Formulations
3D printing represents an innovative Advanced Manufacturing Technology (AMT) that enables the precise production of complex drug formulations and dosage forms. This technology offers the unique capability to create patient-specific dosages, intricate geometric structures, and novel drug delivery systems that are often challenging, if not impossible, to achieve with conventional manufacturing methods.2
For generic manufacturers, 3D printing presents distinct opportunities to develop differentiated products. These products can feature enhanced therapeutic properties, improved patient compliance (e.g., easier-to-swallow shapes, personalized flavors), or novel release characteristics (e.g., multi-layered tablets for controlled release).2 The technology also holds significant promise for the future development of personalized generics, where medications are precisely tailored to individual patient needs, potentially at the point of care.18
This capability is instrumental in shifting the generic paradigm from merely producing a “copy” to creating an “enhanced copy” or a “differentiated product.” Traditionally, generics are defined by their bioequivalence to a brand-name drug, with the primary focus on replication and cost-effectiveness.12 However, 3D printing enables the development of “differentiated products with enhanced therapeutic properties, improved patient compliance, or novel release characteristics”.2 This represents a strategic shift for generic development, moving beyond simple replication to embrace “incremental innovation” or “re-innovation”.2 This allows generic companies to carve out higher-value niches in the market, potentially commanding better market positions and pricing for these “enhanced generics,” thereby expanding their competitive strategies beyond pure cost leadership.
Furthermore, 3D printing exemplifies the convergence of advanced manufacturing and personalized medicine. The technology’s ability to produce “patient-specific dosages” 2 and “customized medications tailored to individual patients’ needs” 18 directly links advanced manufacturing capabilities to the burgeoning field of personalized medicine. This suggests a future where generic drugs are not solely mass-produced commodities but can be customized at the point of care or by specialized compounding facilities. This offers an unprecedented level of patient-centricity for off-patent drugs, with profound implications for individualized patient outcomes and the evolving role of pharmacies and healthcare providers in delivering tailored therapeutic solutions.
2.3 Automation and Robotics in Production
The pharmaceutical industry is increasingly embracing automation to significantly enhance the quality, safety, and efficiency of drug manufacturing processes. Automation directly addresses long-standing challenges such as mitigating human error, reducing variability in production, and ensuring consistent regulatory compliance. Simultaneously, it contributes to improved drug availability and overall cost-effectiveness.27
Robotics, a key component of automation, can perform a wide array of production steps with high precision and repeatability. These include transportation of materials, precise liquid handling, accurate weighing, consistent dispensing, and meticulous inspection of components and finished drug products.27 Such systems are particularly valuable in sensitive environments like formulation and filling, especially for automating visual inspection, performing aseptic manipulations, and executing high-throughput filling procedures.27 The implementation of automation into drug manufacturing systems is highly desirable because it minimizes the human element from production processes, thereby reducing the risk of human error and providing a substantial opportunity to accelerate production times.27
A significant benefit of automated systems is their capacity to provide rapid feedback, enabling dynamic adjustments to meet stringent product specifications in real-time. This capability optimizes efficiency and throughput capabilities within manufacturing facilities.27 Case studies provide tangible evidence of this impact: Antares Pharma, for example, successfully implemented a fluid-filling automation system that substantially improved the yield of finished drugs and cut production costs by up to 60%.27 Similarly, Dyport Laboratories has made a substantial investment in constructing a 100,000-square-foot, fully automated drug manufacturing plant, signaling a strong commitment to leveraging robotics and automation for future production.27
Beyond direct production, AI-powered predictive maintenance plays a crucial role in maximizing operational efficiency. By analyzing real-time sensor data from manufacturing equipment, AI can identify potential issues and predict when equipment is likely to fail before a breakdown occurs.1 This proactive approach helps avoid costly downtime, keeps production running smoothly, and maximizes overall equipment effectiveness and uptime, further contributing to the efficiency and reliability of generic drug manufacturing.
2.4 Bioinformatics and Advanced Analytics
2.4.1 Bioinformatics for Drug Target Identification and Optimization
Bioinformatics, an interdisciplinary field that combines biology, computer science, and statistics, is proving invaluable in expediting various stages of drug development. It leverages computational tools to analyze vast amounts of biological data, including genomics, transcriptomics, proteomics, and metabolomics.28 This analytical power significantly accelerates the identification of potential drug targets, enhances the screening and optimization of drug candidates, and facilitates the detailed characterization of side effects and the prediction of drug resistance.28
By analyzing large biological datasets, researchers can gain a deeper understanding of disease pathogenesis, leading to the discovery of novel diagnostic markers and therapeutic targets.29 This capability is particularly supportive of the development of personalized and precision medicine approaches, even for generic applications, by allowing for a more tailored understanding of drug action and patient response. The ability to identify targets and optimize candidates more quickly and accurately, even for existing generic drugs (e.g., identifying new uses for existing molecules), contributes to the overall acceleration and de-risking of the generic drug development process. This allows generic manufacturers to explore new indications or refine existing formulations with greater efficiency and precision.
2.4.2 Advanced Analytics for Supply Chain Optimization and Market Strategy
Advanced analytics, encompassing tools such as Artificial Intelligence (AI), Machine Learning (ML), and data mining, plays a transformative role in accelerating drug discovery, development, manufacturing, and critically, supply chain optimization.17
In supply chain management, advanced analytics significantly enhances efficiency and resilience. It enables sophisticated demand forecasting and inventory management, allowing pharmaceutical companies to anticipate demand fluctuations and seasonal trends, thereby optimizing inventory levels and ensuring timely delivery of medications. This proactive approach reduces waste and prevents costly stockouts.17 Companies like Merck have demonstrated substantial improvements in their supply chain efficiency, achieving a 95% On-Time In-Full (OTIF) delivery rate through the application of analytics.17 Advanced analytics also provides end-to-end visibility across supply chain activities, including enhanced supplier connectivity and real-time traceability across manufacturing, warehouse, and distribution centers. This comprehensive visibility is crucial for identifying vulnerabilities and adapting to future disruptions.17
The traditional generic drug distribution model relied heavily on a level of global coordination and predictability that no longer consistently exists.31 This has led to a need for data-driven resilience in a volatile global market. Advanced analytics, particularly through the use of Real-World Data (RWD), provides real-time, granular, and actionable insights to map supply chain risks, pinpoint bottlenecks, and respond to delays before they escalate into drug shortages.31 This shifts the industry from a reactive approach to a proactive risk management stance, crucial for ensuring consistent drug availability.
For strategic market entry and competitive intelligence, advanced analytics offers invaluable support. Platforms like DrugPatentWatch provide deep knowledge on pharmaceutical drugs, including patents, suppliers, generics, and formulation details.42 This platform offers competitive intelligence by tracking litigation, patent expirations, and Paragraph IV challenges, which are critical for generic companies to anticipate market shifts and identify entry opportunities.42 By combining such patent landscape analysis with RWD, generic companies can make highly informed decisions regarding patent challenges, optimal market timing, and pricing strategies.31 This enables them to move beyond simple cost competition to a more sophisticated strategic market positioning, leveraging data to gain a competitive advantage. For example, RWD can help measure the real-world effectiveness of generics, navigate fragmented regulatory environments, strengthen domestic drug strategies, and power emergency responses, thereby building public trust through transparent, evidence-backed performance data.31 This comprehensive data-driven approach is essential for future-proofing the generic drug ecosystem against unforeseen disruptions.31
3. Impact on Key Metrics: Cost, Time, and Quality
The integration of advanced technologies across the generic drug development lifecycle is yielding profound impacts on critical industry metrics, fundamentally transforming the economic and operational landscape for generic manufacturers.
3.1 Cost Reduction
Technological advancements are driving significant cost reductions throughout the generic drug development process. Artificial Intelligence (AI) can reduce drug discovery costs by up to 40%.4 Considering that the overall cost of bringing a new drug to market traditionally exceeds $2.5 billion, AI-driven efficiencies promise substantial reductions in this financial burden.4 Generative AI, specifically, has demonstrated the capacity to cut capital costs by an impressive 80% in early design efforts, further enhancing financial efficiency in the initial stages of development.6
In manufacturing and supply chain operations, the application of AI and advanced analytics can lead to considerable savings. These include 5-10% in procurement savings, 10-20% in better conversion costs, and 10-15% in improved cost of quality.17 Automation, as exemplified by Antares Pharma, has shown the ability to cut production costs by up to 60% through fluid-filling automation systems, demonstrating the direct financial benefits of reducing human intervention and optimizing processes.27 Furthermore, the use of virtual bioequivalence (BE) trials, powered by AI, holds the potential to reduce the significant costs associated with extensive human clinical studies, a major expense in generic drug approval.39 These quantifiable cost reductions underscore the strategic imperative for generic manufacturers to adopt these technologies. The ability to significantly lower expenses across the entire lifecycle is crucial for maintaining profitability in a price-sensitive market and for generating capital to fund further research and development into more complex or differentiated generic products.
3.2 Accelerated Timelines
Technological advances are dramatically accelerating development timelines, enabling generic drugs to reach the market much faster. AI can reduce overall drug development timelines from an industry average of 10-15 years to as little as 1-2 years, representing up to a 70% reduction.4 Specifically, the pre-clinical stages alone, which traditionally take 3-6 years, can be compressed to 12-18 months with AI-driven approaches.4 AI has proven capable of identifying promising drug candidates 10 times faster than traditional methods, allowing researchers to screen thousands of molecules in hours instead of months.5
In clinical trials, AI can cut trial duration by up to 10% through optimized design and real-time data analysis.1 Advanced manufacturing techniques, particularly continuous manufacturing, can speed up production from weeks to mere days, significantly reducing lead times.25 The implementation of virtual BE trials further contributes to this acceleration by reducing the need for lengthy and resource-intensive human studies.39 These accelerated timelines translate directly into quicker market entry for generic companies. This allows them to capitalize on patent expirations sooner, capture market share more rapidly, and respond with greater agility to market demands, thereby enhancing their competitive edge in a dynamic industry.
3.3 Enhanced Quality and Consistency
Beyond cost and time, technology is also profoundly enhancing the quality and consistency of generic drug products. AI-driven systems are optimizing pharmaceutical manufacturing by reducing errors and improving product consistency. Real-time analytics allow production lines to adjust dynamically, thereby enhancing both efficiency and quality.1 Advanced manufacturing technologies, such as Continuous Manufacturing (CM) and Process Analytical Technology (PAT), significantly improve product consistency, reduce batch failures, and minimize production variability through real-time monitoring and automated quality control systems.2 These quality improvements not only mitigate regulatory risks but also serve as crucial competitive differentiators in markets where quality concerns have historically impacted confidence in certain generic products.2
AI-powered predictive maintenance identifies potential equipment issues before they lead to costly breakdowns, ensuring continuous production flows and maximizing equipment uptime and efficiency.1 In the formulation stage, AI helps predict the stability of drug formulations under various storage conditions and optimizes drug delivery, contributing to more robust and reliable products.22 These comprehensive quality enhancements are not merely about meeting regulatory standards; they are about building and maintaining public trust in generic medicines and establishing a reputation for reliability and excellence in a highly competitive market.
4. Challenges and Considerations for Technology Adoption
While the transformative potential of technological advancements in generic drug development is immense, their successful adoption is contingent upon navigating several significant challenges related to regulatory frameworks, data security, ethical considerations, and economic hurdles.
4.1 Regulatory Adaptation and Compliance
The rapid pace of technological innovation, particularly in Artificial Intelligence (AI) and Machine Learning (ML), presents a complex landscape for regulatory bodies. The U.S. Food and Drug Administration (FDA) acknowledges the increased use of AI throughout the drug product lifecycle and has issued draft guidance on the use of AI to support regulatory decision-making in drug and biological products.41 However, this evolving regulatory framework introduces several challenges for generic manufacturers.
One central challenge is the validation and verification of adaptive AI/ML models. Traditional validation approaches are often inadequate for algorithms whose behaviors may change over time, necessitating robust mechanisms for tracking and auditing modifications.47 Regulators advocate for “locked” models at the time of validation, with predefined change control plans for any updates, viewing continuous learning models skeptically without such rigorous oversight.47 Another critical area is data integrity, where AI/ML systems must adhere to ALCOA+ principles (attributable, legible, contemporaneous, original, accurate, complete, consistent, enduring, and available) throughout the data pipeline, from training to deployment.47 Black-box algorithms, which obscure their internal workings, pose a significant hurdle to data provenance and auditability, necessitating the implementation of explainable AI (XAI) techniques.47 Furthermore, ensuring transparency and interpretability of complex AI models is crucial for regulatory acceptance, particularly when AI systems are used in decision-making processes related to product quality and safety. Regulators expect manufacturers to understand the logic behind AI predictions and to provide scientific justification.47 This calls for an “Explainability by Design” methodology, where interpretable models are built from the ground up, rather than attempting to explain opaque models retrospectively.47
The regulatory landscape is a dynamic interaction where technological advancements continually push the boundaries of existing frameworks. While technology offers immense benefits in terms of speed and efficiency, its full realization depends on regulators’ ability to adapt and create clear, consistent pathways for approval. This ensures that safety and efficacy standards are maintained without stifling innovation. This highlights the critical need for ongoing dialogue, collaboration, and shared understanding between industry stakeholders and regulatory bodies to foster an environment where technological progress can be safely and effectively integrated into the generic drug development and approval process.
4.2 Data Security, Privacy, and Ethical Concerns
The widespread adoption of AI in pharmaceutical development, particularly within the generic sector, introduces significant data security, privacy, and ethical considerations that must be meticulously addressed. AI systems frequently handle highly sensitive genetic and health information, necessitating robust regulations to protect individual rights and maintain public trust.48 A concerning reality is the high risk of sensitive data exposure to AI tools; a recent study revealed that 83% of pharmaceutical companies lack automated controls to prevent sensitive data from leaking through AI platforms. This means proprietary molecular structures, clinical trial results, and patient records are often inadvertently exposed when employees use general-purpose AI tools.50 Compliance with stringent data protection regulations such as HIPAA (Health Insurance Portability and Accountability Act) and GDPR (General Data Protection Regulation) is therefore paramount.51
Beyond security, several ethical dilemmas arise. Algorithmic bias is a critical issue, as AI models can reflect and perpetuate existing biases present in their training data, potentially leading to inequitable healthcare outcomes.48 Transparency in AI decision-making is essential, as “black box” models, whose internal workings are opaque, hinder trust, particularly in critical areas like drug discovery and genetics.33 The inherent difficulty in deciphering the conclusive derivations of complex AI models necessitates enhanced methodological transparency and uncertainty quantification.33 Furthermore, generic AI models, often trained on broad, non-clinical datasets, can misinterpret domain-specific terminology, abbreviations, or context prevalent in clinical notes and lab reports. This can lead to inaccurate or irrelevant outputs, a phenomenon known as “hallucinating” or the “missing middle” problem, where details in the middle of long texts are overlooked.52 This highlights the need for purpose-built AI models trained on clinical data to ensure accuracy and relevance.52 Ethical considerations also extend to human oversight, fairness in application, and ensuring the safety and data ownership rights of individuals.48
The immense benefits offered by AI in generic drug development come with significant responsibilities. Addressing these challenges is not merely a matter of regulatory compliance but is fundamental to maintaining public trust, preventing potential harm, and ensuring equitable access to medicines. This necessitates a proactive, “ethical-by-design” approach to AI development and deployment, coupled with strong internal governance policies and collaborative efforts across the industry to establish and adhere to clear ethical guidelines.
4.3 Economic and Implementation Hurdles
Despite the clear advantages, the adoption of advanced technologies in the generic drug industry faces substantial economic and implementation hurdles. A primary barrier is the high upfront investment required for advanced manufacturing technologies (AMTs) and the development of robust AI infrastructure.2 While these technologies promise long-term cost reductions and efficiency gains, the initial capital expenditure can be prohibitive, particularly for mid-tier and smaller generic manufacturers who may struggle to compete with larger players.30
Another significant challenge is the need for specialized talent and a fundamental cultural shift within organizations to fully embrace data-driven insights and decision-making.30 The successful integration of AI and advanced analytics requires not only new technical skills but also a willingness to re-evaluate traditional workflows and adopt new methodologies. Recruiting and developing the right analytical talent is a competitive endeavor, and a corporate culture that incentivizes learning new skills and supports data-driven approaches is crucial for success.30
Furthermore, the generic drug market is characterized by intense competition and pervasive pricing pressures.2 This “razor-thin profit margin” environment makes it challenging for generic companies to fund the necessary research and development activities and substantial investments required for technology adoption.2 The cost of developing a generic drug can range from $2 million to $10 million, depending on its complexity, creating significant barriers to entry even before considering advanced technology investments.2
While the long-term return on investment (ROI) for these technologies is evident in terms of cost reduction, accelerated timelines, and quality improvements, the initial capital expenditure and the challenge of acquiring and retaining specialized talent represent significant barriers. This suggests that for broader adoption across the generic industry, particularly for smaller and mid-sized firms, strategic partnerships or outsourced models for analytics and technology implementation may become necessary to catch up with or even leapfrog competitors.30
5. Future Outlook and Strategic Implications
The trajectory of technological advancement suggests a future where generic drug development will be increasingly sophisticated, efficient, and patient-centric. These shifts necessitate proactive strategic responses from industry players and adaptive regulatory frameworks.
5.1 Emerging Trends and Technologies
The future of generic drug development will be profoundly shaped by the continued proliferation and integration of advanced technologies. The Artificial Intelligence (AI) market in pharmaceuticals is projected to experience robust growth, soaring from $1.8 billion in 2023 to an estimated $13.1 billion by 2034.1 This expansion indicates an escalating reliance on AI across the entire drug product lifecycle. Consequently, regulatory submissions incorporating AI components have seen a significant increase, and the FDA is actively developing guidance to support regulatory decision-making based on AI-generated information.41
A key emerging trend is the potential for personalized generics, enabled by technologies such as 3D printing. This allows for the creation of customized medications tailored to individual patients’ specific needs, moving beyond the traditional one-size-fits-all approach to generic production.18 This capability could lead to improved patient outcomes and compliance by optimizing dosage forms and delivery.
Blockchain technology is also being explored for its potential to enhance supply chain security and transparency in the generic drug industry.18 By providing an immutable and traceable record of drug movement, blockchain could help combat counterfeiting, improve recall efficiency, and build greater trust in the supply chain.
The increasing integration of wearable technologies and digital health technologies (DHTs) will revolutionize real-world data (RWD) collection and patient monitoring.41 These devices can provide continuous, granular data on patient health and drug responses, offering invaluable insights for post-market surveillance, optimizing treatment regimens, and even informing future generic drug development. The FDA recognizes the growing integration of AI in areas like DHTs and RWD analytics.41
Furthermore, the generic drug sector is expected to see increased integration with biosimilars and other emerging therapies, such as gene and cell therapies.18 This could involve the development of generic versions of complex biosimilars or the use of generic drugs in combination with these advanced therapeutic modalities, blurring the lines between traditional generics and innovative medicines. These trends indicate a move towards a more sophisticated, data-rich, and patient-centric generic industry, transforming its role in the broader healthcare ecosystem.
5.2 Strategic Imperatives for Generic Manufacturers
To thrive in this evolving technological landscape, generic manufacturers face several strategic imperatives:
- Investment in Research and Development (R&D) for Higher-Value Generics: Given the intense competition and pricing pressures in conventional generic segments, companies must strategically invest in R&D to develop “higher-value generics”.3 This includes complex generics, combination products, and those leveraging advanced formulations or delivery systems, which can command better market positions and pricing.
- Leveraging Digital and Analytics Tools for Agility and Resilience: The industry must embrace digital and analytics tools to enhance operational agility and supply chain resilience.3 This means adopting advanced analytics for demand forecasting, inventory optimization, and real-time visibility across the supply chain to navigate disruptions and ensure consistent supply.17
- Exploring Forward and Backward Integration: To create new revenue streams and reduce costs, generic companies should consider forward and backward integration strategies.3 This could involve greater control over API sourcing or direct engagement with distribution channels, reducing reliance on intermediaries.
- Adopting Advanced Analytics for Competitive Intelligence and Market Entry: Strategic decision-making on market entry and portfolio management will increasingly rely on advanced analytics. Tools like DrugPatentWatch are crucial for competitive intelligence, enabling companies to monitor patent expirations, litigation, and identify generic entry opportunities, allowing for more informed and timely market launches.42
- Prioritizing Data Governance, Security, and Ethical AI Deployment: As data becomes a central asset, robust data governance frameworks, stringent security measures, and an ethical approach to AI deployment are non-negotiable. This is essential not only for regulatory compliance (e.g., HIPAA, GDPR) but also for maintaining patient trust and competitive advantage.51
- Cultivating a Data-Driven Culture and Talent: Companies must foster a culture that embraces data-driven insights and invest in recruiting and developing talent with expertise in AI, ML, and data science. This includes addressing the cultural shift necessary to integrate these technologies effectively across all functions.30
These strategic imperatives represent the actionable steps for generic companies to move beyond a purely cost-driven model to one that embraces innovation, strategic differentiation, and operational excellence, ensuring their continued relevance and success in the pharmaceutical market.
5.3 Role of Regulatory Bodies and Policy Makers
Regulatory bodies and policy makers play a pivotal role in shaping the future of generic drug development by fostering an environment that encourages innovation while safeguarding public health. The U.S. Food and Drug Administration (FDA) has demonstrated proactive engagement through various initiatives, including the development of the Bioequivalence Assessment Mate (BEAM) tool to improve bioequivalence assessment, the Innovative Science and Technology Approaches for New Drugs (ISTAND) program to encourage new drug development tools, and the issuance of draft guidance on the use of Artificial Intelligence (AI) to support regulatory decision-making.3 These initiatives signify a commitment to adapting regulatory processes to accommodate emerging technologies.
A critical responsibility for regulators is to establish clear, consistent, and adaptable ethical and regulatory frameworks for the integration of AI and other advanced technologies.33 This involves addressing complex issues such as the validation of adaptive AI models, ensuring data integrity and lineage, promoting transparency and explainability of AI algorithms, and managing change control for continuously learning systems.47 The development of “Explainability by Design” methodologies and robust mechanisms for tracking AI model modifications will be crucial for regulatory acceptance.47
Furthermore, promoting increased international collaboration on generic approvals can help harmonize standards and accelerate market access for high-quality generic drugs globally.12 Regulators are not merely gatekeepers but are increasingly becoming facilitators of innovation. Their ability to develop clear guidelines, provide scientific and technical assistance, and foster collaborative dialogues with industry stakeholders is essential for the safe and effective adoption of new technologies. Ultimately, this proactive and adaptive regulatory approach will be instrumental in ensuring that the benefits of technological advancements translate into broader patient access to affordable, high-quality medicines worldwide.
6. Conclusions
The analysis unequivocally demonstrates that technological advances are not merely influencing but are fundamentally transforming generic drug development. The integration of Artificial Intelligence (AI) and Machine Learning (ML), alongside advanced manufacturing technologies like Continuous Manufacturing and 3D printing, and the pervasive application of automation and advanced analytics, is proving indispensable for the future viability and growth of the generic pharmaceutical industry.
These innovations are driving profound improvements across key metrics:
- Cost Reduction: Substantial savings are being realized in drug discovery (up to 40%), early design efforts (up to 80% capital cost reduction), manufacturing (up to 60% production cost reduction), and clinical development (up to $25 billion in savings), enabling generic manufacturers to navigate razor-thin margins and reinvest in innovation.1
- Accelerated Timelines: Development cycles are being dramatically compressed, with overall drug development timelines potentially reduced by up to 70%, and production times shortened from weeks to days. This allows for quicker market entry, rapid response to patent expirations, and enhanced competitive advantage.4
- Enhanced Quality and Consistency: AI-driven systems, real-time monitoring in continuous manufacturing, and automated quality control are significantly reducing errors, improving product consistency, and minimizing production variability. This not only ensures regulatory compliance but also builds critical trust and differentiates generic products in the market.1
Beyond these quantifiable benefits, technology is enabling the development of more complex and higher-value generic formulations, including biosimilars and potentially personalized generics. This represents a strategic shift from simple replication to incremental innovation, allowing generic companies to carve out new market niches and contribute more broadly to patient outcomes by offering enhanced therapeutic properties and improved patient compliance.2
However, the full realization of this transformative potential hinges on effectively addressing the significant challenges that accompany technology adoption. Navigating evolving regulatory frameworks, ensuring robust data security and patient privacy, and resolving complex ethical dilemmas related to algorithmic bias and transparency are paramount. Furthermore, overcoming the substantial upfront economic investments and cultivating a data-driven organizational culture with specialized talent are critical implementation hurdles.
In conclusion, the future of generic drug development lies in its strategic embrace of these technological advancements. This requires sustained investment in R&D, a commitment to digital transformation, and proactive collaboration between industry stakeholders and regulatory bodies. By collectively fostering an environment that promotes responsible innovation, the generic pharmaceutical industry can continue its vital mission of providing affordable, high-quality, and increasingly sophisticated medicines, thereby enhancing global patient access and contributing significantly to public health.
Works cited
- AI in Pharma and Biotech: Market Trends 2025 and Beyond – Coherent Solutions, accessed July 24, 2025, https://www.coherentsolutions.com/insights/artificial-intelligence-in-pharmaceuticals-and-biotechnology-current-trends-and-innovations
- Innovations in Generic Drug Manufacturing and Formulation: Transforming Products and Reducing Costs – DrugPatentWatch, accessed July 24, 2025, https://www.drugpatentwatch.com/blog/innovations-in-generic-drug-manufacturing-and-formulation-transforming-products-and-reducing-costs/
- The Future of Generic Drug Development: Emerging Technologies and Approaches, accessed July 24, 2025, https://www.drugpatentwatch.com/blog/the-future-of-generic-drug-development-emerging-technologies-and-approaches/
- How AI Is Already Changing Drug Development – DrugPatentWatch, accessed July 24, 2025, https://www.drugpatentwatch.com/blog/how-ai-is-already-changing-drug-development/
- AI in Drug Discovery: How AI Is Accelerating Pharma Research (Key Stats) | PatentPC, accessed July 24, 2025, https://patentpc.com/blog/ai-in-drug-discovery-how-ai-is-accelerating-pharma-research-key-stats
- How Generative AI Is Reducing Drug Discovery Timelines by 70% | by PrajnaAI | Medium, accessed July 24, 2025, https://prajnaaiwisdom.medium.com/how-generative-ai-is-reducing-drug-discovery-timelines-by-70-e86d58f7c780
- Generic Drug Development Accelerated by Machine Learning and Automation: Complex Formulations Like Biosimilars Now Possible – GeneOnline, accessed July 24, 2025, https://www.geneonline.com/generic-drug-development-accelerated-by-machine-learning-and-automation-complex-formulations-like-biosimilars-now-possible/
- Report: 2023 U.S. Generic and Biosimilar Medicines Savings Report, accessed July 24, 2025, https://accessiblemeds.org/resources/reports/2023-savings-report-2/
- Estimating Cost Savings from New Generic Drug Approvals in 2022 | September, 2024 – FDA, accessed July 24, 2025, https://www.fda.gov/media/182435/download
- Sandoz CEO: Switzerland could save millions on medicines – SWI swissinfo.ch, accessed July 24, 2025, https://www.swissinfo.ch/eng/business/sandoz-ceo-switzerland-could-save-millions-on-medicines/48860712
- Understanding the Pharmaceutical Drug Development Life Cycle, accessed July 24, 2025, https://www.drugzone.com/blog/understanding-the-pharmaceutical-drug-development-life-cycle
- Obtaining Generic Drug Approval in the United States – DrugPatentWatch, accessed July 24, 2025, https://www.drugpatentwatch.com/blog/obtaining-generic-drug-approval-in-the-united-states/
- Timeline: Generic medicines in the US – USP, accessed July 24, 2025, https://www.usp.org/our-impact/generics/timeline-of-generics-in-us
- Predicting patent challenges for small-molecule drugs: A cross-sectional study – PMC, accessed July 24, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC11867330/
- AI-driven innovations in pharmaceuticals: optimizing drug discovery and industry operations, accessed July 24, 2025, https://pubs.rsc.org/en/content/articlehtml/2025/pm/d4pm00323c
- Harnessing Artificial Intelligence in Generic Formulation Development and Life Cycle Management – A Comprehensive Review, accessed July 24, 2025, https://healthinformaticsjournal.al-makkipublisher.com/index.php/hij/article/view/45
- 8 Use Cases For Data Analytics In Pharmaceutical Industry, accessed July 24, 2025, https://www.polestarllp.com/blog/analytics-in-pharmaceutical-companies
- The Future of Generic Drugs – Number Analytics, accessed July 24, 2025, https://www.numberanalytics.com/blog/future-generic-drugs-development
- Artificial Intelligence in Drug Formulation and Delivery: Benefits, Trends, and Future Perspectives – ResearchGate, accessed July 24, 2025, https://www.researchgate.net/publication/391571513_Artificial_Intelligence_in_Drug_Formulation_and_Delivery_Benefits_Trends_and_Future_Perspectives
- AI-Driven Drug Discovery: A Comprehensive Review | ACS Omega, accessed July 24, 2025, https://pubs.acs.org/doi/10.1021/acsomega.5c00549
- The Potential of Artificial Intelligence in Pharmaceutical Innovation: From Drug Discovery to Clinical Trials – MDPI, accessed July 24, 2025, https://www.mdpi.com/1424-8247/18/6/788
- How Artificial Intelligence (AI) and Machine Learning (ML) Concepts are Transforming Generic Pharmaceuticals, accessed July 24, 2025, https://witii.us/how-artificial-intelligence-ai-and-machine-learning-ml-concepts-are-transforming-generic-pharmaceuticals/
- Artificial Intelligence in Pharmaceutical Technology and Drug Delivery Design – PMC, accessed July 24, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC10385763/
- Generative AI in the pharmaceutical industry: Moving from hype to reality – McKinsey, accessed July 24, 2025, https://www.mckinsey.com/industries/life-sciences/our-insights/generative-ai-in-the-pharmaceutical-industry-moving-from-hype-to-reality
- New manufacturing and analytical technologies – USP, accessed July 24, 2025, https://www.usp.org/our-science/new-manufacturing-and-analytics-technologies
- The Future of Pharma: Continuous Manufacturing – Number Analytics, accessed July 24, 2025, https://www.numberanalytics.com/blog/future-of-pharma-continuous-manufacturing
- Automation in Drug Manufacturing: Ensuring Quality and Safety – Research Inventions Journals, accessed July 24, 2025, https://rijournals.com/wp-content/uploads/2025/03/RIJSES-51-2025-P10.pdf
- pmc.ncbi.nlm.nih.gov, accessed July 24, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC11865229/#:~:text=Bioinformatic%20analysis%20can%20expedite%20the,and%20prediction%20of%20drug%20resistance.
- The role and application of bioinformatics techniques and tools in drug discovery – PMC, accessed July 24, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC11865229/
- Advanced analytics fuel tomorrow’s commercial strategy for drugs and devices – PwC, accessed July 24, 2025, https://www.pwc.com/us/en/industries/health-industries/health-research-institute/commercial-pharma-analytics.html
- Decoding the Generic Supply Chain: Why Pharma Data Analytics Hold the Key to Saving the Generic Drug Ecosystem | PurpleLab®, accessed July 24, 2025, https://purplelab.com/pharma-data-analytics-generic-drugs/
- Optimizing the Generic Drug Supply Chain: Strategies for Success – DrugPatentWatch, accessed July 24, 2025, https://www.drugpatentwatch.com/blog/optimizing-the-generic-drug-supply-chain-strategies-for-success/
- Regulating the Use of AI in Drug Development: Legal Challenges and Compliance Strategies, accessed July 24, 2025, https://www.fdli.org/2025/07/regulating-the-use-of-ai-in-drug-development-legal-challenges-and-compliance-strategies/
- (PDF) Influence of artificial intelligence in modern pharmaceutical formulation and drug development – ResearchGate, accessed July 24, 2025, https://www.researchgate.net/publication/379425899_Influence_of_artificial_intelligence_in_modern_pharmaceutical_formulation_and_drug_development
- Harnessing AI To Speed Up Drug Formulation – Merck Group, accessed July 24, 2025, https://www.merckgroup.com/en/research/science-space/envisioning-tomorrow/precision-medicine/harnessing-ai-to-speed-up-drug-formulation.html
- Machine learning driven acceleration of biopharmaceutical formulation development using Excipient Prediction Software (ExPreSo) | bioRxiv, accessed July 24, 2025, https://www.biorxiv.org/content/10.1101/2025.02.12.637685v1.full-text
- Harnessing Artificial Intelligence in Generic Formulation Development and Life Cycle Management – A Comprehensive Review, accessed July 24, 2025, https://healthinformaticsjournal.al-makkipublisher.com/index.php/hij/article/download/45/49/536
- Machine learning driven bioequivalence risk assessment at an early stage of generic drug development – PubMed, accessed July 24, 2025, https://pubmed.ncbi.nlm.nih.gov/39490606/
- Artificial Intelligence Meets Bioequivalence: Using Generative Adversarial Networks for Smarter, Smaller Trials – MDPI, accessed July 24, 2025, https://www.mdpi.com/2504-4990/7/2/47
- Physiologically Based Pharmacokinetics Modeling in Biopharmaceutics: Case Studies for Establishing the Bioequivalence Safe Space for Innovator and Generic Drugs | Request PDF – ResearchGate, accessed July 24, 2025, https://www.researchgate.net/publication/362030821_Physiologically_Based_Pharmacokinetics_Modeling_in_Biopharmaceutics_Case_Studies_for_Establishing_the_Bioequivalence_Safe_Space_for_Innovator_and_Generic_Drugs
- Artificial Intelligence for Drug Development – FDA, accessed July 24, 2025, https://www.fda.gov/about-fda/center-drug-evaluation-and-research-cder/artificial-intelligence-drug-development
- DrugPatentWatch has been a game-changer for our business, accessed July 24, 2025, https://www.drugpatentwatch.com/
- DrugPatentWatch | Software Reviews & Alternatives – Crozdesk, accessed July 24, 2025, https://crozdesk.com/software/drugpatentwatch
- DrugPatentWatch Pricing, Features, and Reviews (Jul 2025) – Software Suggest, accessed July 24, 2025, https://www.softwaresuggest.com/drugpatentwatch
- Effects of Generic Entry on Market Shares and Prices of Originator Drugs – PubMed Central, accessed July 24, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC12209137/
- Generic Drug Entry Timeline: Predicting Market Dynamics After Patent Loss, accessed July 24, 2025, https://www.drugpatentwatch.com/blog/generic-drug-entry-timeline-predicting-market-dynamics-after-patent-loss/
- Regulatory Perspectives for AI/ML Implementation in Pharmaceutical GMP Environments – PMC – PubMed Central, accessed July 24, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC12195787/
- Ethical Dilemmas and Privacy Issues in Emerging Technologies: A Review – MDPI, accessed July 24, 2025, https://www.mdpi.com/1424-8220/23/3/1151
- Ethical Considerations Emerge from Artificial Intelligence (AI) in Biotechnology – PMC, accessed July 24, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC11910024/
- AI Data Security The 83% Compliance Gap Facing Pharmaceutical Companies, accessed July 24, 2025, https://www.clinicalleader.com/doc/ai-data-security-the-compliance-gap-facing-pharmaceutical-companies-0001
- AI, Data Privacy & Compliance in Pharma: 4 Key Takeaways from Industry Experts, accessed July 24, 2025, https://www.pulsepoint.com/blog/ai-data-privacy-pharma-marketing
- Why generic AI fails pharma and how purpose-built AI is revolutionizing drug development, accessed July 24, 2025, https://discover-pharma.com/why-generic-ai-fails-pharma-and-how-purpose-built-ai-is-revolutionizing-drug-development/
- The future of clinical trials and drug development: 2050 – PMC – PubMed Central, accessed July 24, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC10259497/