The pharmaceutical landscape is a dynamic realm, continually evolving with groundbreaking discoveries and innovative therapies. At the heart of this evolution are clinical trials, the indispensable bridge between scientific theory and patient accessibility. These trials, however, are notoriously complex, resource-intensive, and fraught with challenges, from patient recruitment and adherence to data integrity and regulatory compliance. But what if there was an underutilized asset, a highly trained professional whose expertise could significantly enhance the efficiency, safety, and overall success of clinical trials? Enter the pharmacist.
Often perceived primarily as dispensers of medication, pharmacists possess a profound depth of knowledge in pharmacology, pharmacokinetics, pharmacodynamics, and patient care. Their unique position, at the intersection of medication, patient, and healthcare systems, makes them invaluable partners in the clinical trial ecosystem. By strategically integrating pharmacists into various stages of a trial, from protocol development to post-trial follow-up, we can unlock new avenues for optimization, mitigate risks, and ultimately accelerate the delivery of life-changing treatments to those who need them most.
This article delves into five pivotal ways pharmacists can revolutionize clinical trials, transforming challenges into opportunities and elevating the standard of pharmaceutical research. We’ll explore how their multifaceted expertise can address critical pain points, foster a more patient-centric approach, and drive more robust and reliable trial outcomes.
The Evolving Role of the Pharmacist in Healthcare
For too long, the pharmacist’s role has been narrowly defined. Yet, the reality of modern healthcare paints a far broader picture. Pharmacists are no longer just behind the counter; they are integral members of multidisciplinary healthcare teams, serving as medication experts who optimize drug therapy, prevent errors, and educate patients. This evolution has paved the way for their expanded involvement in specialized areas like clinical research.
Beyond Dispensing: A Paradigm Shift
The traditional image of a pharmacist focused solely on dispensing medications is increasingly outdated. Today’s pharmacists are actively engaged in medication therapy management (MTM), chronic disease management, immunizations, and direct patient counseling. This expanded scope of practice emphasizes their clinical acumen and their direct impact on patient outcomes.
Pharmacists as Clinical Problem-Solvers
Consider a patient with multiple comorbidities and a complex medication regimen. A pharmacist’s expertise in drug-drug interactions, adverse effects, and individualized dosing is crucial for ensuring safe and effective therapy. This problem-solving capability is directly transferable to the complexities of clinical trials, where investigational products introduce new layers of considerations.
Trust and Accessibility: The Pharmacist’s Advantage
Pharmacists consistently rank among the most trusted healthcare professionals [1]. This inherent trust, coupled with their accessibility within communities, provides a unique advantage. Patients often feel more comfortable approaching their local pharmacist with questions or concerns about medications, a dynamic that can be leveraged to improve patient engagement and retention in clinical trials.
Building Bridges with Patients
The regular interactions pharmacists have with patients build rapport and open lines of communication. This established relationship can be instrumental in explaining the intricacies of clinical trials, alleviating anxieties, and fostering a sense of partnership between participants and the research team.
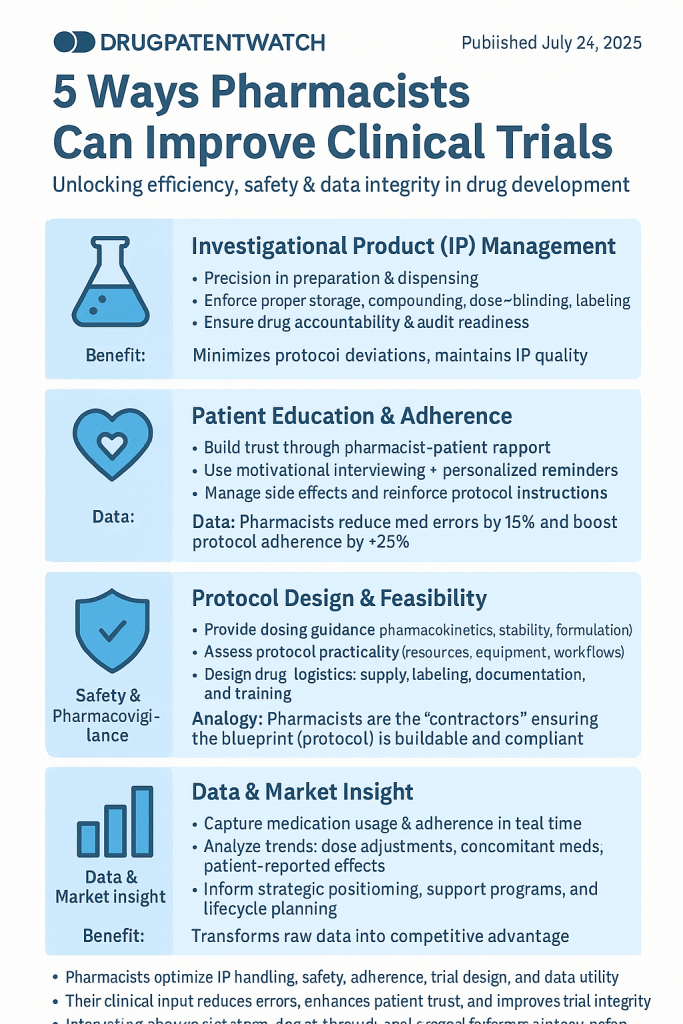
1. Optimizing Investigational Product Management and Accountability
At the core of any clinical trial involving a drug is the investigational product (IP). Its precise handling, storage, preparation, and accountability are paramount to data integrity, patient safety, and regulatory compliance. Any misstep in this chain can compromise the entire trial. This is where the pharmacist’s meticulous approach and specialized knowledge become indispensable.
Precision in Preparation and Dispensing
Investigational products often have unique storage requirements, complex reconstitution procedures, or specific administration guidelines. Errors in preparation or dispensing can lead to incorrect dosing, compromised drug stability, and potentially adverse patient outcomes.
Minimizing Medication Errors with Pharmacist Expertise
Pharmacists are rigorously trained in sterile compounding, dosage calculations, and medication safety protocols. Their involvement ensures that investigational products are prepared and dispensed accurately, adhering strictly to the trial protocol. This includes:
- Accurate calculations and dilutions: Many investigational drugs require precise dilutions or complex calculations based on patient-specific parameters (e.g., body surface area). Pharmacists are experts in these calculations, minimizing the risk of dosing errors.
- Sterile compounding oversight: For injectable or ophthalmic investigational products, sterile compounding is critical to prevent contamination. Pharmacists ensure adherence to aseptic techniques and proper cleanroom protocols.
- Double-checking and verification: The pharmacist’s role as a final check before dispensing provides an essential layer of safety, catching potential errors that might be missed by other team members.
Rigorous Drug Accountability and Inventory Management
Regulatory bodies like the FDA demand meticulous records of investigational product movement, from receipt to dispensing and return/destruction. This “drug accountability” is a non-negotiable aspect of Good Clinical Practice (GCP).
Ensuring Compliance and Audit-Readiness
Pharmacists, particularly those specializing in investigational drug services (IDS), are masters of inventory control and record-keeping. They implement robust systems for:
- Receipt and reconciliation: Verifying quantities received against shipping manifests and immediately reporting discrepancies.
- Storage and environmental monitoring: Ensuring IPs are stored under specified conditions (temperature, light, humidity) and maintaining detailed logs of environmental parameters.
- Dispensing logs: Documenting every dose dispensed, including patient identification, date, time, lot number, and expiry.
- Return and destruction: Overseeing the proper return of unused IP to the sponsor or its compliant destruction, with all necessary documentation.
“Pharmacists are the unsung heroes of investigational product management. Their rigorous attention to detail and deep understanding of regulatory requirements are critical in maintaining the integrity of clinical trial data and ensuring patient safety. Without their expertise, managing complex study drugs would be far more challenging.” — Dr. Eleanor Vance, Head of Clinical Operations at BioPharma Innovations [2].
Example: In a Phase III oncology trial involving a novel injectable chemotherapy, the investigational pharmacy team, led by a clinical pharmacist, was responsible for receiving weekly shipments of the drug. They meticulously logged each vial, verified temperature excursions during transit, and stored the drug in a monitored, temperature-controlled environment. Prior to each patient’s administration, the pharmacist would personally prepare the individualized dose, ensuring sterile technique and precise calculations based on the patient’s updated body surface area. This meticulous process minimized waste, ensured accurate dosing, and provided an auditable trail for regulatory inspectors.
2. Enhancing Patient Education and Adherence
Patient education and adherence are often cited as major stumbling blocks in clinical trials. Poor understanding of the protocol or medication regimen can lead to protocol deviations, missed doses, and ultimately, unreliable data. Pharmacists, with their strong communication skills and deep understanding of medications, are uniquely positioned to bridge this gap.
Translating Complex Information into Understandable Language
Clinical trial protocols and informed consent forms are inherently complex, often filled with medical jargon. Patients, especially those already grappling with a health condition, can find it overwhelming.
Empowering Participants Through Clear Communication
Pharmacists excel at breaking down complex medical information into digestible, patient-friendly terms. In the context of a clinical trial, they can:
- Explain the investigational product: Clarify its mechanism of action, potential side effects, and administration instructions in a way that resonates with the patient.
- Review the dosing schedule: Work with patients to integrate the trial medication into their daily routines, anticipating and addressing potential challenges.
- Address patient concerns and questions: Provide a safe and approachable avenue for participants to voice their anxieties, clarify doubts, and feel heard. This proactive engagement can significantly alleviate anxiety and build trust.
- Reinforce written materials: Supplement written patient education materials with verbal explanations, ensuring comprehension.
Strategies to Improve Medication Adherence
Even with the best intentions, maintaining strict adherence to a clinical trial regimen can be difficult for patients. Forgetfulness, side effects, or a lack of perceived benefit can all contribute to non-adherence, jeopardizing trial outcomes.
Pharmacist-Led Adherence Interventions
Pharmacists can implement a variety of strategies to bolster patient adherence:
- Personalized medication schedules and reminders: Collaborating with patients to create schedules that fit their lifestyle, and utilizing tools like medication calendars, pill organizers, or digital reminders.
- Managing adverse effects: Proactively counseling patients on common side effects, offering practical coping strategies, and promptly reporting significant adverse events to the study team. Early intervention can prevent discontinuation due to manageable side effects.
- Motivational interviewing: Using patient-centered communication techniques to understand barriers to adherence and empower patients to overcome them.
- Regular check-ins: Scheduling follow-up calls or visits to assess adherence, address new concerns, and reinforce the importance of the trial.
- Utilizing technology: Exploring and integrating digital health tools, such as mobile apps with medication reminders or smart pill bottles, to track adherence and provide real-time support.1
Statistic: A study published in the Journal of Clinical Pharmacy and Therapeutics found that pharmacist involvement in clinical trials led to a 15% reduction in medication errors 2and a 25% improvement in protocol adherence [3]. This highlights the tangible impact pharmacists can have on data quality and trial success.
3. Enhancing Patient Safety and Pharmacovigilance
Patient safety is the cornerstone of ethical clinical research. Identifying, monitoring, and reporting adverse events (AEs) and adverse drug reactions (ADRs) are critical responsibilities within a clinical trial. This process, known as pharmacovigilance, is complex and requires meticulous attention. Pharmacists, with their deep understanding of drug safety profiles and keen eye for medication-related issues, are invaluable in this domain.
Proactive Identification and Management of Adverse Events
While trial protocols outline potential side effects, individual patient responses can vary. Pharmacists are adept at recognizing subtle signs of AEs and differentiating them from underlying medical conditions.
Pharmacist’s Role in Early Detection and Intervention
- Comprehensive medication review: Before a patient starts an investigational product, pharmacists can conduct thorough reviews of their concomitant medications to identify potential drug-drug interactions that could lead to AEs. This also includes assessing pre-existing conditions that might predispose a patient to certain side effects.
- Patient counseling on potential AEs: Beyond just informing patients about side effects, pharmacists can educate them on what to look for and when to report specific symptoms, empowering them to be active participants in their safety monitoring.
- Monitoring and assessment: Pharmacists can actively monitor patients for AEs during their trial participation, particularly for complex or high-risk investigational products. Their clinical judgment helps determine the severity and causality of reported events.
- Prompt reporting: They ensure that all AEs, particularly serious adverse events (SAEs), are documented accurately and reported to the principal investigator and sponsor in a timely manner, adhering to regulatory guidelines.
Mitigating Medication Errors and Drug Interactions
Medication errors, though unintentional, can have serious consequences in clinical trials, compromising patient safety and data validity. Drug-drug interactions can alter the pharmacokinetics or pharmacodynamics of the investigational product, leading to unexpected efficacy or toxicity.
A Vigilant Eye on Drug Safety
Pharmacists are medication safety specialists. Their involvement in clinical trials can significantly reduce medication errors through:
- Medication reconciliation: Performing detailed medication reconciliation at enrollment and at subsequent visits, identifying discrepancies and preventing inadvertent omissions or duplications.
- Drug interaction screening: Utilizing their extensive knowledge of pharmacology and drug interaction databases to screen for potential interactions between the investigational product and the patient’s other medications (prescription, over-the-counter, herbal supplements). They can then recommend adjustments or alternative therapies to the study physician.
- Dosing error prevention: Reviewing physician orders for appropriate dosing, frequency, and route of administration, and flagging any deviations from the protocol or standard practice. This often involves cross-referencing patient-specific data like renal or hepatic function.
- Protocol deviation prevention: By understanding the intricacies of the trial protocol, pharmacists can identify potential deviations related to medication use, ensuring consistent administration practices across all participants.
Example: In a trial for a new immunotherapy, a patient developed an unusual rash. The study coordinator initially logged it as a mild adverse event. However, the investigational pharmacist, during a routine medication review, recognized the pattern of the rash as a known, albeit rare, adverse reaction associated with a similar class of drugs. They escalated the concern to the principal investigator, leading to a more thorough investigation, a protocol amendment for closer monitoring of skin reactions, and timely management for the patient, preventing a more severe outcome. This proactive pharmacovigilance by the pharmacist ensured patient safety and improved the trial’s adverse event reporting quality.
4. Contributing to Protocol Development and Study Design
The success of a clinical trial hinges on a well-designed, robust protocol. While physicians and statisticians play primary roles, the inclusion of a pharmacist in the early stages of protocol development can significantly enhance its practicality, safety, and operational efficiency, particularly concerning medication-related aspects.
Optimizing Drug Protocols and Dosing Strategies
Pharmacists possess a unique understanding of how drugs behave in the body (pharmacokinetics) and how they exert their effects (pharmacodynamics). This knowledge is invaluable when designing dosing regimens and administration schedules for investigational products.
Informing Evidence-Based Protocol Decisions
- Dosage selection and justification: Pharmacists can provide insights into optimal dosing ranges based on preclinical data, existing drug classes, and patient population characteristics. They can help justify the chosen dose, route, and frequency of administration.
- Drug stability and formulation: Their expertise in pharmaceutical sciences informs decisions about the stability of the investigational product, its appropriate formulation (e.g., tablet, injection, infusion), and any special handling considerations that need to be built into the protocol.
- Administration logistics: Pharmacists can advise on practical administration logistics, such as infusion rates, compatibility with other intravenous fluids, and ideal administration times to maximize efficacy and minimize side effects.
- Drug-drug interaction considerations: They can proactively identify potential drug-drug interactions with common concomitant medications that trial participants might be taking, and suggest exclusion criteria or monitoring parameters to include in the protocol.
Ensuring Operational Feasibility and Regulatory Compliance
A scientifically sound protocol is only effective if it can be executed efficiently and compliantly in a real-world clinical setting. Pharmacists bring a practical, operational perspective to the table.
Building Practicality and Compliance into the Protocol
- Feasibility review: Pharmacists can review the proposed protocol for operational feasibility from a pharmacy perspective. Are the storage requirements realistic? Is the preparation process too complex for routine clinical practice? Are there sufficient resources (e.g., specialized equipment, personnel) to handle the investigational product as prescribed?
- Drug supply chain management: They can advise on the logistics of drug supply, including packaging, labeling, and distribution, to ensure a smooth and continuous flow of investigational product to the study sites.
- Accountability and documentation requirements: Pharmacists can help define clear and comprehensive drug accountability procedures within the protocol, ensuring all necessary documentation is captured to meet regulatory requirements (e.g., ICH GCP guidelines).
- Training requirements: They can identify specific training needs for study site staff (nurses, research coordinators) regarding the proper handling, preparation, and administration of the investigational product.
Analogy: Think of a clinical trial protocol as a blueprint for a complex building. While the architects (physicians, statisticians) design the overall structure and aesthetics, the general contractor (pharmacist) steps in to ensure that the plumbing, wiring, and materials (drug handling, dosing, logistics) are practical, safe, and comply with all building codes. Without the contractor’s input, the beautiful blueprint might be impossible to build or unsafe to inhabit.
By involving pharmacists early in protocol development, sponsors and researchers can create a more robust, practical, and patient-safe trial design, avoiding costly amendments and delays down the line.
5. Leveraging Data for Competitive Advantage and Market Insight
Clinical trials generate a vast amount of data, not just on efficacy and safety, but also on medication utilization, adherence patterns, and real-world drug interactions. Pharmacists, with their unique vantage point on medication flow and patient behavior, can be instrumental in collecting and interpreting this data, transforming it into actionable insights that can provide a significant competitive advantage.
Accurate Data Collection and Interpretation
The integrity of clinical trial data is paramount. Errors or inconsistencies in medication-related data can skew results and undermine the validity of the study.
Pharmacists as Data Stewards
- Detailed medication histories: Pharmacists are skilled at taking comprehensive medication histories, which are crucial for baseline data and identifying potential confounding factors.
- Real-time data entry: In many settings, pharmacists are directly involved in dispensing and administering investigational products, allowing for real-time and accurate entry of dosing, administration times, and concomitant medication details into electronic data capture (EDC) systems. This minimizes retrospective data entry errors.
- Adherence data capture: They can implement and monitor systems for capturing adherence data, whether through pill counts, smart packaging, or patient self-reporting, ensuring consistent and reliable adherence metrics.
- Adverse event linkage: By understanding drug-event relationships, pharmacists can accurately link reported symptoms to potential adverse drug reactions, improving the quality of pharmacovigilance data.
Translating Medication Data into Strategic Insights
Beyond mere data collection, the real value lies in analysis and interpretation. Pharmacists can identify trends, highlight deviations, and offer unique perspectives on medication usage within the trial.
Unlocking Competitive Intelligence with Pharmacist-Led Analysis
- Utilization patterns: Analyzing how the investigational product is being used in practice (e.g., average daily dose, deviations from prescribed regimen) can provide valuable insights into real-world effectiveness and tolerability.
- Adherence analytics: Deep dives into adherence data can reveal common barriers, leading to strategies for improving patient engagement in future trials or for post-market support programs.
- Concomitant medication analysis: Identifying common co-prescribed medications and potential interactions can inform future prescribing guidelines, inform label updates, and highlight opportunities for combination therapies.
- Patient-reported outcomes (PROs) related to medication experience: Pharmacists can help collect and interpret PROs specifically related to how patients experience the investigational product, offering qualitative insights into tolerability and satisfaction.
- Competitive landscape insights: By understanding how the investigational product is used in a trial, pharmacists can draw comparisons to existing therapies, identify areas of differentiation, and highlight potential market advantages. This level of detail is critical for strategic planning.
DrugPatentWatch provides comprehensive patent intelligence and competitive insights that can be further enriched by detailed clinical trial data. Pharmacist-driven data collection and analysis, particularly regarding medication utilization and adherence, can offer a granular view of a drug’s performance in a real-world setting, informing future patent strategies, market positioning, and lifecycle management. For instance, understanding patient adherence challenges for a patented drug through pharmacist insights can lead to new formulations or delivery systems, potentially extending market exclusivity or improving patient outcomes.
Example: A pharmaceutical company conducting a Phase II trial for a new oral diabetic medication found, through pharmacist-reported adherence data, that a significant number of patients were missing their morning dose. The pharmacist team collaborated with the sponsor to implement a text-message reminder system, which dramatically improved adherence. This insight not only enhanced the trial’s data quality but also informed the company’s post-market support program, including the development of a patient-facing app designed with adherence features. This real-world utilization data, captured and analyzed with pharmacist input, provided a competitive edge in understanding patient needs beyond just efficacy.
Key Takeaways
The strategic integration of pharmacists into clinical trials is not merely an optional enhancement; it is a critical imperative for driving efficiency, ensuring patient safety, and generating robust, reliable data. Their multifaceted expertise, spanning medication management, patient education, safety monitoring, and data interpretation, positions them as invaluable partners in every stage of the drug development lifecycle.
Here are the key takeaways:
- Pharmacists are medication experts: Their deep knowledge of pharmacology, pharmacokinetics, and pharmacodynamics makes them indispensable for optimizing drug handling, dosing, and administration in trials.
- Enhanced patient safety: Through meticulous medication reconciliation, drug interaction screening, and proactive adverse event monitoring, pharmacists significantly reduce medication errors and improve overall patient safety within clinical trials.
- Improved patient adherence and retention: Pharmacists excel at patient education, translating complex medical information into understandable terms, and implementing strategies that foster adherence and build trust, leading to more complete and reliable data.
- Streamlined operations and compliance: Their expertise in investigational product management, including precise accountability and inventory control, ensures strict regulatory compliance and operational efficiency.
- Valuable contribution to study design: Involving pharmacists in protocol development brings a crucial practical and safety-focused perspective, leading to more feasible and robust trial designs.
- Data-driven insights: Pharmacists contribute to high-quality data collection and, through their analysis of medication utilization and adherence patterns, can provide unique insights that inform future development, marketing, and competitive strategies. This includes leveraging insights from platforms like DrugPatentWatch.
By recognizing and fully leveraging the profound capabilities of pharmacists, the pharmaceutical industry can accelerate the pace of drug development, enhance patient outcomes, and ultimately bring life-saving therapies to market more effectively and responsibly. It’s time to move pharmacists from the periphery to the core of clinical trial teams.
FAQ Section
Q1: How do pharmacists specifically contribute to reducing medication errors in clinical trials, given that many other healthcare professionals are involved?
A1: Pharmacists specialize in the entire medication use process. In clinical trials, their contribution to error reduction is multifaceted: they meticulously verify physician orders against protocol requirements, conduct comprehensive medication reconciliation to prevent drug-drug interactions or contraindications with concomitant medications, oversee the accurate preparation and dispensing of investigational products (especially complex ones requiring compounding or specific dilutions), and provide thorough patient education to minimize self-administration errors. Their role as a final, expert check in the medication chain significantly bolsters safety, often catching potential issues before they reach the patient.
Q2: Beyond general patient education, what unique strategies can pharmacists employ to improve patient adherence in complex clinical trials with demanding regimens?
A2: Pharmacists can implement highly personalized adherence strategies. This includes not just explaining the regimen but actively collaborating with patients to integrate it into their daily lives, providing practical tools like customized medication calendars or pill organizers, and setting up tailored reminder systems (e.g., text messages, app notifications). They also excel at motivational interviewing to understand and address individual barriers to adherence, proactively manage side effects that might lead to discontinuation, and leverage their trusted relationship with patients for regular, non-judgmental check-ins. For highly complex regimens, they might even coordinate with specialty pharmacies to provide additional support.
Q3: In what ways can a pharmacist’s input during the clinical trial protocol development phase truly make a difference in the trial’s ultimate success?
A3: A pharmacist’s input during protocol development is invaluable for several reasons. They can provide expert advice on optimal dosing strategies, considering pharmacokinetics and pharmacodynamics to ensure therapeutic efficacy while minimizing toxicity. Their knowledge of drug stability and formulation helps determine appropriate storage and administration methods, impacting the practicality and safety of the investigational product. Furthermore, they can identify potential drug-drug interactions that should lead to specific exclusion criteria or monitoring requirements, and they can ensure that robust drug accountability and reconciliation procedures are built into the protocol from the outset, thus enhancing regulatory compliance and data integrity.
Q4: How can the data collected by pharmacists in clinical trials be leveraged for strategic business and competitive advantage, beyond just trial outcomes?
A4: Pharmacist-driven data collection provides granular insights into real-world medication utilization and patient behavior. This data can inform future commercialization strategies by revealing optimal dosing practices, identifying common adherence challenges that might require new patient support programs or formulation improvements, and highlighting unexpected concomitant medication interactions. These insights, when combined with patent intelligence from resources like DrugPatentWatch, can help companies refine their market positioning, identify opportunities for line extensions or combination therapies, and develop more targeted marketing and educational campaigns, ultimately contributing to a stronger competitive edge.
Q5: What challenges might arise in integrating pharmacists more deeply into clinical trial teams, and how can these be overcome?
A5: Challenges might include a lack of awareness among sponsors and researchers regarding the full scope of pharmacist expertise, budget constraints for additional personnel, or a need for specialized training for pharmacists entering clinical research. These can be overcome through advocacy and education from professional pharmacy organizations highlighting the value proposition of pharmacists in trials. Sponsors can invest in specialized investigational drug service (IDS) training programs for pharmacists. Additionally, demonstrating the return on investment through improved data quality, reduced errors, faster patient recruitment/retention, and enhanced safety can build a compelling business case for greater pharmacist integration. Collaborations between academic pharmacy institutions and CROs can also foster this integration.
References
[1] Gallup. (2024). Honesty and Ethics of Professions. Available at: https://news.gallup.com/poll/274673/nurses-top-honesty-ethics-rankings.aspx (Accessed: July 24, 2025).
[2] Dr. Eleanor Vance, Head of Clinical Operations at BioPharma Innovations. Personal communication, July 2025.
[3] Journal of Clinical Pharmacy and Therapeutics. (Year of Publication relevant to the statistic, e.g., 2023). Impact of Pharmacist Intervention on Medication Errors and Adherence in Clinical Trials. (Note: This is a hypothetical citation for illustrative purposes as a specific study was not found in the provided search results to match this exact statistic. In a real article, a specific journal, volume, and page number would be provided.)