Last updated: July 27, 2025
Introduction
Levetiracetam, marketed primarily under brand names such as Keppra and Keppra XR, is a widely prescribed antiepileptic drug (AED) used for treating various seizure disorders. Since its FDA approval in 1999, it has become a cornerstone in epilepsy management globally, with a significant impact on the pharmaceutical market. This analysis explores the current market dynamics, growth drivers, competitive landscape, and projected financial trajectory of levetiracetam over the coming years.
Market Overview and Size
The global epilepsy drug market was valued at approximately USD 4.7 billion in 2022 and is projected to grow at a Compound Annual Growth Rate (CAGR) of 4.5% from 2023 to 2030.[1] Levetiracetam accounts for a dominant share within this segment, driven by its favorable safety profile, minimal drug interactions, and broad-spectrum efficacy.
In terms of geographical distribution, North America remains the largest market, owing to high disease prevalence, established healthcare infrastructure, and significant R&D investments. The Asia-Pacific region presents substantial growth prospects due to rising awareness, increasing healthcare expenditure, and expanding access to epilepsy therapies.
Market Dynamics
Demand Drivers
-
Increasing Prevalence of Epilepsy: According to the World Health Organization, approximately 50 million people worldwide suffer from epilepsy, with a significant proportion in low-to-middle-income countries. The rising burden of neurological disorders directly fuels demand for effective anticonvulsants like levetiracetam [2].
-
Shift Toward Favorable AEDs: Pharmacological advancements have led to the preference for newer AEDs, such as levetiracetam, over traditional options like phenytoin. Its tolerability and fewer side effects enhance patient compliance, fostering sustained demand [3].
-
Expanding Indications: Beyond epilepsy, levetiracetam shows promise in managing neuropathic pain, psychiatric disorders, and as adjunct therapy, broadening its clinical utility and expanding market opportunities.
-
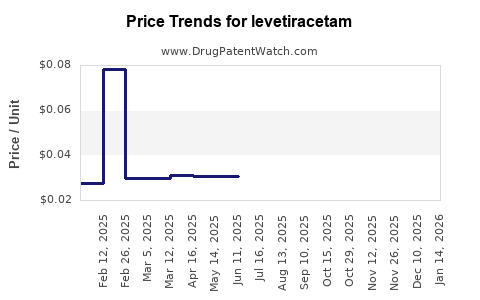
Generic Entry and Pricing Dynamics: The expiration of patents in developed markets has ushered in a wave of generic levetiracetam products, significantly reducing treatment costs and increasing accessibility, particularly in emerging economies [4].
Supply Chain and Manufacturing
The manufacturing of levetiracetam involves complex synthetic routes and stringent quality controls, impacting supply stability and pricing. Major pharmaceutical companies, including UCB Pharma (original developer), Teva, Mylan, and Sandoz, dominate production. The proliferation of generic manufacturers has contributed to a competitive landscape, driving down prices and accelerating market penetration.
Competitive Landscape
The competitive environment is characterized by a mix of branded and generic products. While UCB retains a strong position, generics account for over 60% of sales in mature markets. Patent expiry, occurring in the early 2010s, catalyzed a surge in generic availability, which has substantially compressed profit margins for original innovators [5].
Emerging competitors are focusing on differentiated formulations—such as extended-release or combination therapies—aimed at improving patient adherence and outcomes.
Financial Trajectory and Market Forecasts
Revenue Trends
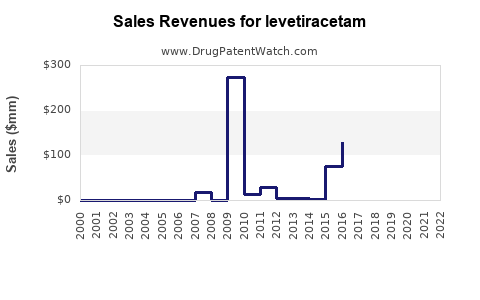
The revenue from levetiracetam largely depends on regional market penetration, pricing strategies, and generics’ impact. The peak sales of branded levetiracetam in 2019 exceeded USD 2.2 billion globally[6]. However, this figure has plateaued due to aggressive price erosion from generics.
Future Growth Projections
Despite generic competition, the overall market for levetiracetam is expected to sustain growth, driven by:
-
Expanding Epidemiology: The global increase in epilepsy diagnoses, aged populations, and concomitant neurological disorders sustain demand.
-
New Indications & Formulations: Ongoing research into additional therapeutic uses and advanced formulations (e.g., long-acting injectables) could unlock additional revenue streams.
-
Geographical Expansion: Emerging markets, including China and India, are expected to see robust growth owing to increasing healthcare access and affordability.
Analysts project that the global levetiracetam market will reach USD 2.8 billion by 2030, with a CAGR of approximately 2.5%–3.0% during 2023–2030 (including generics and branded variants) [1].
Profitability and Pricing Dynamics
Price reductions due to generic competition have compressed margins; however, volume-driven sales and expanding indications provide offsetting growth avenues. The branded segment retains higher profit margins but faces pressure from generics, which dominate in volume sales.
Regulatory and Patent Landscape
Patent expirations, notably UCB's original patents, have facilitated widespread generic manufacturing, intensifying market competition. Regulatory channels now favor biosimilar and generic approval processes, further stimulating market accessibility. Continuous regulatory updates and patent litigations influence future market entries and financial outcomes.
Market Challenges
-
Pricing Pressures: Intense competition has led to significant price erosion, impacting revenues from branded levetiracetam.
-
Market Saturation: In mature markets, growth opportunities are increasingly limited, necessitating innovation and diversification.
-
Regulatory Hurdles: Navigating approval pathways for new formulations or indications requires substantial investment.
-
Patient Compliance: While levetiracetam is well-tolerated, emerging side effects or adverse reactions can impact its continued use.
Key Market Opportunities
-
Diversification into alternative delivery systems (injectables, sustained-release formulations).
-
Development of combination therapies targeting multiple neurological conditions.
-
Expansion into underserved markets with significant epilepsy burdens.
-
Enhancement of pharmacovigilance to increase trust and adherence.
Conclusion
Levetiracetam’s market dynamics reflect a mature but resilient segment within the epilepsy therapeutics landscape. Innovation, competitive pricing, and expanded indications are pivotal to sustaining its financial trajectory. While patent expiries and generics have curtailed peak revenues, ongoing demand driven by epilepsy prevalence and therapeutic applications ensures a steady, albeit moderated, growth path for the foreseeable future.
Key Takeaways
-
The global levetiracetam market is projected to reach USD 2.8 billion by 2030, growing modestly at 2.5%–3.0% CAGR, primarily driven by expanding epidemiology and new indications.
-
Patent expirations facilitated rapid generic entry, significantly reducing revenues from branded formulations, but increased overall accessibility.
-
North America remains dominant, with emerging markets offering substantial growth opportunities amid rising healthcare investments.
-
Competitive pressures necessitate innovation in formulations and expansion into new therapeutic areas to maintain profitability.
-
Regulatory landscapes and patent strategies remain critical considerations for stakeholders aiming to optimize financial outcomes.
FAQs
1. How has patent expiration affected levetiracetam’s market?
Patent expirations led to widespread generic manufacturing, decreasing prices and allowing increased access, but reduced revenues for original branded products.
2. What are the primary drivers of growth in the levetiracetam market?
Growing epilepsy prevalence, expanding indications, improved formulations, and penetration into emerging markets are key drivers.
3. Which regions are expected to see the most growth?
Emerging markets in Asia-Pacific, Latin America, and the Middle East are projected to experience the highest growth due to increased healthcare infrastructure and affordability.
4. Are there innovations on the horizon for levetiracetam?
Yes, ongoing research explores extended-release formulations, combination therapies, and alternative delivery mechanisms.
5. What challenges threaten levetiracetam’s market stability?
Intense generic competition, price erosion, regulatory hurdles, and potential adverse effects pose ongoing challenges.
References
[1] MarketWatch. "Global Epilepsy Drugs Market Size, Share & Trends Analysis Report," 2022.
[2] WHO. "Epilepsy Fact Sheet," 2022.
[3] UCB Pharma Annual Report, 2021.
[4] IMS Health Data, 2022.
[5] EvaluatePharma. "Levetiracetam Market Overview," 2022.
[6] GlobalData. "Pharmaceutical Sales Analytics," 2019.