LEVETIRACETAM Drug Patent Profile
✉ Email this page to a colleague
When do Levetiracetam patents expire, and what generic alternatives are available?
Levetiracetam is a drug marketed by Am Regent, Epic Pharma Llc, Eugia Pharma, Fresenius Kabi Usa, Hainan Poly Pharm, Hikma Farmaceutica, Hospira Inc, Jubilant Generics, Micro Labs, MSN, Mylan Labs Ltd, Prinston Inc, Sagent Pharms, Sun Pharm Inds Ltd, Xgen Pharms, Actavis Mid Atlantic, Ajenat Pharms, Alembic, Amneal Pharms, Apotex Inc, Aurobindo Pharma, Bionpharma, Chartwell Molecular, Hetero Labs Ltd Iii, Hikma, Lupin Ltd, Pharm Assoc, Pharmobedient Cnsltg, Quagen, Strides Pharma, Taro, Tolmar, Actavis Elizabeth, Actavis Labs Fl Inc, Adaptis, Aiping Pharm Inc, Anda Repository, Apotex, Aurobindo Pharma Usa, Chartwell Rx, Hisun Pharm Hangzhou, Lotus Pharm Co Ltd, Overseas, Ph Health, Pharmadax Inc, Rouses Point Pharms, Sandoz, Sciegen Pharms Inc, Sun Pharm, Sun Pharm Industries, Teva Pharms, Torrent Pharms Ltd, Accord Hlthcare, Alkem Labs Ltd, China Resources, Fosun Pharma, Granules, Ingenus Pharms Llc, Invagen Pharms, Lupin, Mpp Pharma, Mylan, Orbion Pharms, Oxford Pharms, Rising, Torrent Pharms, Viwit Pharm, Watson Labs Inc, Zhejiang Jingxin, Zydus Pharms Usa Inc, B Braun Medical, Baxter Hlthcare Corp, Caplin, Gland, Hq Spclt Pharma, and Nexus. and is included in one hundred and three NDAs.
The generic ingredient in LEVETIRACETAM is levetiracetam. There are thirty-five drug master file entries for this compound. Eighty-six suppliers are listed for this compound. Additional details are available on the levetiracetam profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Levetiracetam
A generic version of LEVETIRACETAM was approved as levetiracetam by MYLAN on November 4th, 2008.
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for LEVETIRACETAM?
- What are the global sales for LEVETIRACETAM?
- What is Average Wholesale Price for LEVETIRACETAM?
Summary for LEVETIRACETAM
| US Patents: | 0 |
| Applicants: | 76 |
| NDAs: | 103 |
| Finished Product Suppliers / Packagers: | 73 |
| Raw Ingredient (Bulk) Api Vendors: | 1 |
| Clinical Trials: | 245 |
| Patent Applications: | 4,412 |
| Drug Prices: | Drug price information for LEVETIRACETAM |
| Drug Sales Revenues: | Drug sales revenues for LEVETIRACETAM |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for LEVETIRACETAM |
| What excipients (inactive ingredients) are in LEVETIRACETAM? | LEVETIRACETAM excipients list |
| DailyMed Link: | LEVETIRACETAM at DailyMed |
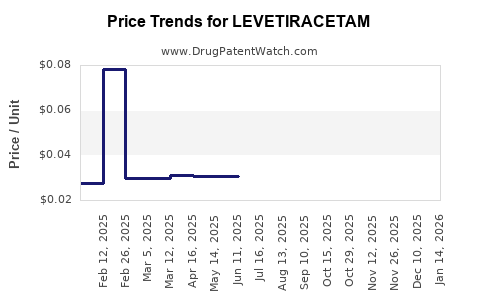
See drug prices for LEVETIRACETAM
Recent Clinical Trials for LEVETIRACETAM
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Nebraska Methodist Health System | PHASE3 |
| Ayal A. Aizer, MD | PHASE2 |
| Tanta University | PHASE3 |
Pharmacology for LEVETIRACETAM
| Physiological Effect | Decreased Central Nervous System Disorganized Electrical Activity |
Anatomical Therapeutic Chemical (ATC) Classes for LEVETIRACETAM
Paragraph IV (Patent) Challenges for LEVETIRACETAM
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| KEPPRA XR | Extended-release Tablets | levetiracetam | 1000 mg | 022285 | 2 | 2011-01-07 |
| KEPPRA | Tablets | levetiracetam | 1000 mg | 021035 | 1 | 2007-01-24 |
US Patents and Regulatory Information for LEVETIRACETAM
EU/EMA Drug Approvals for LEVETIRACETAM
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| UCB Pharma SA | Keppra | levetiracetam | EMEA/H/C/000277Keppra is indicated as monotherapy in the treatment of partial-onset seizures with or without secondary generalisation in patients from 16 years of age with newly diagnosed epilepsy.Keppra is indicated as adjunctive therapy:in the treatment of partial-onset seizures with or without secondary generalisation in adults, children and infants from one month of age with epilepsy;in the treatment of myoclonic seizures in adults and adolescents from 12 years of age with juvenile myoclonic epilepsy;in the treatment of primary generalised tonic-clonic seizures in adults and adolescents from 12 years of age with idiopathic generalised epilepsy. | Authorised | no | no | no | 2000-09-29 | |
| Pfizer Europe MA EEIG | Levetiracetam Hospira | levetiracetam | EMEA/H/C/002783Levetiracetam Hospira is indicated as monotherapy in the treatment of partial onset seizures with or without secondary generalisation in adults and adolescents from 16 years of age with newly diagnosed epilepsy.Levetiracetam Hospira is indicated as adjunctive therapyin the treatment of partial onset seizures with or without secondary generalisation in adults, adolescents and children from 4 years of age with epilepsy.in the treatment of myoclonic seizures in adults and adolescents from 12 years of age with Juvenile Myoclonic Epilepsy.in the treatment of primary generalised tonic-clonic seizures in adults and adolescents from 12 years of age with Idiopathic Generalised Epilepsy.Levetiracetam Hospira concentrate is an alternative for patients when oral administration is temporarily not feasible. | Authorised | yes | no | no | 2014-01-07 | |
| Accord Healthcare S.L.U. | Levetiracetam Accord | levetiracetam | EMEA/H/C/002290Levetiracetam is indicated as monotherapy in the treatment of partial-onset seizures with or without secondary generalisation in patients from 16 years of age with newly diagnosed epilepsy.Levetiracetam is indicated as adjunctive therapy:in the treatment of partial-onset seizures with or without secondary generalisation in adults, children and infants from one month of age with epilepsy;in the treatment of myoclonic seizures in adults and adolescents from 12 years of age with juvenile myoclonic epilepsy;in the treatment of primary generalised tonic-clonic seizures in adults and adolescents from 12 years of age with idiopathic generalised epilepsy. | Authorised | yes | no | no | 2011-10-03 | |
| Pharmathen S.A. | Matever | levetiracetam | EMEA/H/C/002024Matever is indicated as monotherapy in the treatment of partial-onset seizures with or without secondary generalisation in patients from 16 years of age with newly diagnosed epilepsy.Matever is indicated as adjunctive therapy:in the treatment of partial-onset seizures with or without secondary generalisation in adults, children and infants from one month of age with epilepsy;in the treatment of myoclonic seizures in adults and adolescents from 12 years of age with juvenile myoclonic epilepsy;in the treatment of primary generalised tonic-clonic seizures in adults and adolescents from 12 years of age with idiopathic generalised epilepsy. | Authorised | yes | no | no | 2011-10-03 | |
| Actavis Group PTC ehf | Levetiracetam Actavis | levetiracetam | EMEA/H/C/002355Levetiracetam Actavis is indicated as monotherapy in the treatment of partial-onset seizures with or without secondary generalisation in patients from 16 years of age with newly diagnosed epilepsy.Levetiracetam Actavis is indicated as adjunctive therapy:in the treatment of partial-onset seizures with or without secondary generalisation in adults, children and infants from one month of age with epilepsy;in the treatment of myoclonic seizures in adults and adolescents from 12 years of age with juvenile myoclonic epilepsy;in the treatment of primary generalised tonic-clonic seizures in adults and adolescents from 12 years of age with idiopathic generalised epilepsy. | Authorised | yes | no | no | 2011-10-03 | |
| Actavis Group PTC ehf | Levetiracetam Actavis Group | levetiracetam | EMEA/H/C/002305Levetiracetam Actavis Group is indicated as monotherapy in the treatment of partial-onset seizures with or without secondary generalisation in patients from 16 years of age with newly diagnosed epilepsy.Levetiracetam Actavis Group is indicated as adjunctive therapy:in the treatment of partial-onset seizures with or without secondary generalisation in adults, children and infants from 1 month of age with epilepsy;in the treatment of myoclonic seizures in adults and adolescents from 12 years of age with juvenile myoclonic epilepsy;in the treatment of primary generalised tonic-clonic seizures in adults and adolescents from 12 years of age with idiopathic generalised epilepsy. | Authorised | yes | no | no | 2011-12-04 | |
| Sun Pharmaceutical Industries Europe B.V. | Levetiracetam Sun | levetiracetam | EMEA/H/C/002051Levetiracetam Sun is indicated as monotherapy in the treatment of partial-onset seizures with or without secondary generalisation in patients from 16 years of age with newly diagnosed epilepsy.Levetiracetam Sun is indicated as adjunctive therapy:in the treatment of partial-onset seizures with or without secondary generalisation in adults and children from four years of age with epilepsy;in the treatment of myoclonic seizures in adults and adolescents from 12 years of age with juvenile myoclonic epilepsy;in the treatment of primary generalised tonic-clonic seizures in adults and adolescents from 12 years of age with idiopathic generalised epilepsy.Levetiracetam Sun concentrate is an alternative for patients when oral administration is temporarily not feasible. | Authorised | yes | no | no | 2011-12-14 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
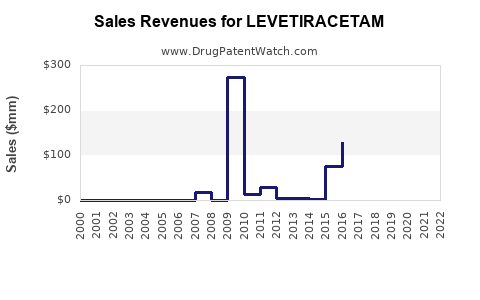
Market Dynamics and Financial Trajectory for Levetiracetam
More… ↓
