I. Introduction: Navigating the Landscape of Pharmaceutical Patents with the FDA Orange and Purple Books
Patent information holds a position of paramount importance within the pharmaceutical industry. It significantly influences drug development pathways, determines market entry timelines for competing products, and shapes overall intellectual property strategies for both innovator and generic or biosimilar drug manufacturers. Accessing and effectively utilizing this information is crucial for stakeholders across the pharmaceutical and legal sectors. The FDA’s Approved Drug Products with Therapeutic Equivalence Evaluations, more commonly known as the Orange Book, and the Lists of Licensed Biological Products with Reference Product Exclusivity and Biosimilarity or Interchangeability Evaluations, referred to as the Purple Book, serve as the primary resources for obtaining this vital data.1
The Orange Book focuses on small-molecule drugs, which are typically chemically synthesized and have well-defined structures.1 It provides a wealth of information regarding these drugs, including their patent status and regulatory exclusivities.3 In contrast, the Purple Book centers on biological products, also known as biologics or large-molecule drugs, which are derived from living organisms and often possess complex structures.4 This publication primarily details reference product exclusivity and evaluations of biosimilarity or interchangeability, with patent information being a more recent addition.5
The creation and evolution of these resources are rooted in significant legislative frameworks. The Hatch-Waxman Act of 1984 established a regulatory pathway for the approval of generic drugs and introduced the requirement for patent listing in the Orange Book.6 This act aimed to balance incentivizing pharmaceutical innovation with facilitating the entry of lower-cost generic medicines upon patent expiration.7 Similarly, the Biologics Price Competition and Innovation Act (BPCIA) of 2010 created an abbreviated approval pathway for biosimilar products and laid the groundwork for the information provided in the Purple Book.8 Recent legislative changes, such as the Orange Book Transparency Act and the Purple Book Continuity Act, further underscore the ongoing efforts to enhance the utility and transparency of these resources.10
In today’s highly competitive pharmaceutical landscape, the strategic importance of the Orange and Purple Books cannot be overstated. The increasing prevalence of generic and biosimilar drugs has made accurate and timely patent research more critical than ever.12 Pharmaceutical companies rely on these resources to identify opportunities for generic or biosimilar development, to analyze the patent landscape surrounding innovator products, and to formulate effective intellectual property and market entry strategies.14 Legal professionals also utilize these books extensively in matters of patent litigation and regulatory compliance.1
II. Decoding the FDA Orange Book for Patent and Exclusivity Insights
- Understanding the Scope and Purpose of the Orange Book:
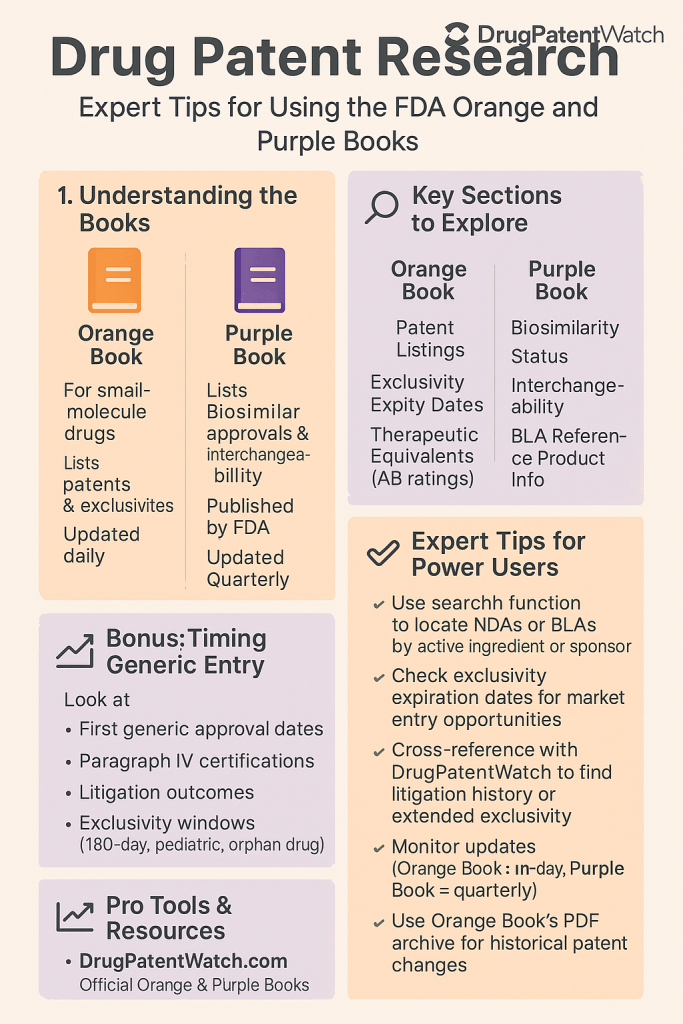
The official title of the Orange Book is “Approved Drug Products with Therapeutic Equivalence Evaluations”.3 This publication serves as a comprehensive guide to drug products that have been approved by the FDA based on their demonstrated safety and effectiveness, as mandated by the Federal Food, Drug, and Cosmetic Act (FD&C Act).3 A key aspect of the Orange Book is the inclusion of patent and exclusivity information pertaining to these approved drugs.3 Additionally, it provides therapeutic equivalence evaluations for multisource prescription drug products, which are crucial for generic substitution practices.16
The FDA publishes and updates the Orange Book regularly to reflect the dynamic nature of the pharmaceutical market. Monthly supplements and an annual edition ensure that stakeholders have access to the most current information.3 Furthermore, new generic drug approvals are generally updated on a daily basis, providing timely intelligence for generic manufacturers and pharmacies.18 These updates encompass various changes, including NDA approvals, exclusivity grants, alterations in firm names, active ingredients, product discontinuations, and modifications to drug strengths, dosage forms, routes of administration, therapeutic equivalence codes, and trade names.19 The Orange Book is accessible in multiple formats to cater to different user needs, including an online searchable database, downloadable PDF publications, and data files.3
The FDA’s commitment to maintaining an up-to-date Orange Book underscores the significance of providing timely information on approved drugs and their intellectual property status. This reflects the continuous evolution within the pharmaceutical industry and the corresponding changes in the patent and exclusivity landscape. The therapeutic equivalence codes listed in the Orange Book have a direct impact on the substitutability of generic drugs for their brand-name counterparts by pharmacists. These codes are often integrated into state pharmacy laws, highlighting the Orange Book’s role in facilitating the use of more affordable generic medications.16
- In-Depth Guide to Patent Listings: Types, Use Codes, and Expiration:
New drug application (NDA) holders bear the responsibility of submitting patent information to the FDA for listing in the Orange Book. This requirement applies to patents that claim the drug substance (the active ingredient), the drug product (its formulation or composition), or a specific method of using the drug for an indication that has received FDA approval.12 This obligation is triggered upon the submission of an NDA and extends to certain supplemental NDAs (sNDAs) that seek approval for changes such as a new dosage form, a different route of administration, a modification in strength, or a switch from prescription to over-the-counter status.22
The submission of patent information involves specific FDA forms. Form FDA 3542a is used for pre-approval submissions, while Form FDA 3542 is utilized for post-approval information.22 For patents that exist at the time of NDA or sNDA submission, Form 3542a is submitted initially, followed by a resubmission on Form 3542 within 30 days of the drug’s approval.22 If a patent is issued while the application is still under review, Form 3542a must be submitted within 30 days of the patent’s issuance. Similarly, for patents granted after the drug has already received approval, Form 3542 must be submitted within 30 days of the patent being issued.22 The Orange Book listing for each patent includes its number, the date it is scheduled to expire, and a patent use code.6
Patent Use Codes are alphanumeric identifiers, typically starting with a “U-” followed by a unique number, that provide a concise description of the specific approved method of using the drug that is covered by the patent.24 These codes are invaluable for generic drug applicants. By understanding the particular uses that are protected by patents, generic manufacturers can identify opportunities to potentially “carve out” those specific uses from the labeling of their generic product. This strategy, known as a section viii statement, allows the generic drug to be approved for the remaining, non-patented indications.25 The FDA provides a comprehensive “(https://www.accessdata.fda.gov/scripts/cder/ob/results_patent.cfm)” to aid in the interpretation of these codes.3 (Note: Users should verify the accessibility of this link.) The patent expiration dates listed in the Orange Book are particularly significant for generic drug manufacturers as they provide a crucial timeline for planning their market entry strategies.6
It is important to note that the Orange Book generally does not include certain types of patents. Patents covering manufacturing processes, the drug’s packaging, metabolites, intermediates involved in synthesis, uses that have not been approved by the FDA, or certain polymorphs of the drug substance are typically excluded.21 However, there are exceptions to this rule. Patents that cover finished dosage forms such as metered aerosols, capsules, metered sprays, gels, and pre-filled syringes are generally eligible for listing if they directly claim the final product.27 Additionally, patents on polymorphs can be listed if the company holding the NDA provides certification along with test data demonstrating that the patented polymorph will perform in the same manner as the drug product described in the original NDA.6
The patent listing process within the Orange Book is a cornerstone of the regulatory framework governing pharmaceutical intellectual property. It aims to foster transparency regarding the patents protecting approved drugs, with a specific focus on claims that directly pertain to the drug product and its authorized uses. The FDA’s role in listing these patents is primarily ministerial, meaning the agency relies on the information provided by the NDA holder.23 Patent use codes play a vital role in enabling generic drug applicants to employ the “skinny label” approach. By precisely identifying the patented uses, generic companies can tailor their labeling to avoid infringing these patents, potentially leading to earlier market entry for their products.24 While the Orange Book serves as a valuable resource, its limitations regarding the types of patents listed necessitate that generic drug manufacturers conduct broader patent searches beyond this publication to gain a complete understanding of potential infringement risks, especially concerning aspects like manufacturing processes.23
- Exploring Regulatory Exclusivities: Definitions, Durations, and Strategic Implications:
Beyond patent protection, the FDA grants certain regulatory exclusivities that provide a limited period of protection from new market competition for approved drugs.12 These exclusivities operate independently of patent rights and are granted if specific statutory requirements are met upon drug approval.28 The purpose of these exclusivities is to incentivize various forms of pharmaceutical innovation.29
Several types of exclusivities exist, each with its own duration and criteria. Five-Year New Chemical Entity (NCE) exclusivity is granted to drugs containing an active moiety that has not been previously approved by the FDA.12 This exclusivity generally prevents the submission of any Abbreviated New Drug Application (ANDA) or a 505(b)(2) application for a drug containing the same active moiety for a period of five years from the date of the NCE’s approval.12 Three-Year New Clinical Studies exclusivity may be granted when an application or supplement contains reports of new clinical investigations (other than bioavailability studies) that were essential for the approval of a new use for a previously approved drug product.12 This new use could involve changes in strength, dosage form, route of administration, or indication.12 Seven-Year Orphan Drug exclusivity is provided for drugs designated and approved to treat rare diseases or conditions affecting fewer than 200,000 people in the United States.12 This exclusivity prevents the approval of any other application for the same drug for the same orphan-protected use during the seven-year period.12 Six-Month Pediatric exclusivity can be added to existing patents or exclusivities associated with a brand-name drug if the sponsor conducts pediatric clinical studies in response to a written request from the FDA.12 The GAIN (Generating Antibiotic Incentives Now) exclusivity generally provides an additional five years of exclusivity added to certain other exclusivity periods for a drug product that has been granted a “Qualified Infectious Disease Product” designation by the FDA.12 The 180-Day exclusivity may be granted to the first generic applicant(s) to submit a substantially complete ANDA that contains a challenge to a patent listed in the Orange Book.12 Finally, Competitive Generic Therapy (CGT) exclusivity of 180 days may be granted to an applicant for a drug product for which there is inadequate generic competition.28
The FDA maintains a “(https://www.accessdata.fda.gov/scripts/cder/ob/results_exclusivity.cfm)” that provides further details on the various types of exclusivities.3 (Note: Users should verify the accessibility of this link as it was previously reported as inaccessible.) The expiration dates for these exclusivities are explicitly listed in the Orange Book.6 It is also important to note that pediatric exclusivity, when granted, can extend the life of any existing patents and exclusivities associated with the drug by an additional six months.12
Regulatory exclusivities serve as important strategic tools to encourage specific types of pharmaceutical innovation. The incentive provided by these periods of market protection can be particularly crucial for the development of treatments for rare diseases or new chemical entities.29 However, the granting of exclusivity can also result in a delay in the market entry of generic drugs, even after the patents on the brand-name drug have expired.28 Therefore, a thorough understanding of the different types of exclusivities and their potential impact is essential for both innovator and generic drug companies when formulating their drug development and market entry strategies.23 Innovator companies often strive to secure all applicable exclusivities to maximize their period of market exclusivity, while generic companies must carefully track these periods to accurately predict when they will be able to launch their generic versions.33
- Expert Strategies for Effective Patent Research Using the Orange Book:
To conduct effective patent research using the Orange Book, several expert strategies can be employed. Researchers can begin by searching the Orange Book database using either the proprietary name (brand name) or the active ingredient of the drug of interest. This will quickly yield a list of any patents and exclusivities associated with that particular drug.3 Once a drug is identified, carefully analyze the patent listings. Note the patent numbers, their corresponding expiration dates, and the specific type of patent, such as whether it covers the drug substance, the drug product, or a method of use.6
Particular attention should be paid to the Patent Use Codes. These codes provide valuable insights into the precise uses of the drug that are protected by patents. Understanding these codes is crucial for generic drug developers as it can help them identify potential opportunities to “carve out” patented uses from their generic product’s label, thus potentially avoiding patent infringement.24 Researchers should also thoroughly review the exclusivity data provided in the Orange Book. Identify the specific type of exclusivity that has been granted and note its expiration date. This information is vital for determining the earliest possible date for generic market entry, as exclusivities can sometimes extend beyond patent expiration.12
It is also beneficial to monitor the Orange Book for any patent delistings. If a patent owner requests a patent to be delisted, it may indicate that the patent has been invalidated or is no longer considered enforceable.6 For more in-depth analysis, the FDA provides downloadable Orange Book data files. These comprehensive datasets can be utilized for more extensive research and trend analysis.3 To gain a better understanding of the Orange Book’s scope, history, and recent updates, it is advisable to review the Orange Book Preface and any related guidance documents issued by the FDA.16 Finally, if there are any questions or concerns regarding the accuracy or relevance of patent information listed in the Orange Book, researchers should consult the Patent Listing Dispute List, which outlines any ongoing disputes and their status.3 By employing these strategies, researchers can effectively leverage the wealth of information contained within the FDA Orange Book for comprehensive drug patent research.
III. Unlocking Patent and Exclusivity Information in the FDA Purple Book
- Understanding the Scope and Purpose of the Purple Book for Biological Products:
The official title of the Purple Book is “Lists of Licensed Biological Products with Reference Product Exclusivity and Biosimilarity or Interchangeability Evaluations”.34 This publication serves as a comprehensive resource for biological products that have been licensed under the Public Health Service (PHS) Act.4 Its primary purpose is to provide information on whether a biological product has been granted reference product exclusivity and whether it has been evaluated and determined to be biosimilar to or interchangeable with a previously licensed reference product.5
Unlike the Orange Book, which was initially a print publication, the Purple Book is primarily available as a searchable online database.36 This database is updated on a monthly basis to reflect any new approvals or changes in the status of licensed biological products.38 The Purple Book encompasses information on all FDA-licensed biological products, including those regulated by the Center for Drug Evaluation and Research (CDER) and the Center for Biologics Evaluation and Research (CBER).34 The transition to a searchable online database has significantly enhanced the accessibility and utility of the Purple Book, allowing users to efficiently find information on a wide range of biological products, including biosimilars and their corresponding reference products.35
The FDA’s development of the Purple Book reflects the increasing significance of biological products in treating various diseases and the growing market for biosimilars. By providing a centralized source of information on reference product exclusivity and biosimilarity evaluations, the Purple Book aims to facilitate the development and approval of biosimilar products, ultimately promoting competition and potentially lowering healthcare costs.34
- Reference Product Exclusivity and its Impact on Biosimilar Development:
A key feature of the Purple Book is the information it provides regarding reference product exclusivity. Under the BPCIA, a reference biological product is typically granted a period of 12 years of exclusivity from the date of its first licensure.5 During this exclusivity period, a 351(k) application for a biosimilar product referencing that specific biological product cannot be effectively approved by the FDA.5 This exclusivity period includes any pediatric exclusivity that may have been granted to the reference product.5 The Purple Book explicitly lists the date of first licensure for reference products, as well as the date on which the reference product exclusivity is scheduled to expire.5 However, it is important to note that the Purple Book does not include information about orphan drug exclusivity periods for biological products.5
Reference product exclusivity plays a critical role in the development of biosimilars. The 12-year exclusivity period provides a substantial window of market protection for innovator biological products, allowing them to recoup the significant investments made in their research and development before facing competition from biosimilar versions.13 Biosimilar companies closely monitor the expiration dates of reference product exclusivity listed in the Purple Book. These dates serve as crucial milestones, indicating the earliest point at which they can potentially gain approval and enter the market with their biosimilar products.15 The expiration of this exclusivity is often the primary trigger for biosimilar manufacturers to file their applications with the FDA.13
- Recent Advancements in Patent Transparency within the Purple Book:
Historically, the Purple Book lacked comprehensive patent information, a significant difference compared to the Orange Book. However, recent legislative changes have aimed to enhance patent transparency for biological products. The Purple Book Continuity Act of 2019, enacted as part of the Consolidated Appropriations Act of 2021, mandates the inclusion of certain patent information in the Purple Book.37 Specifically, reference product sponsors are now required to submit to the FDA lists of patents that they have identified as potentially being infringed by a biosimilar product during the “patent dance” process.8 This submission must occur within 30 days of providing the list to the biosimilar applicant and should include the patent number and its corresponding expiration date.38 The FDA, in turn, publishes these patent lists in the searchable Purple Book database, and this information is updated on a monthly basis.38 It is important to note that the FDA’s role in publishing this patent information is ministerial, meaning the agency does not independently assess the validity or applicability of the listed patents.41
These advancements in patent transparency represent a significant step forward in providing biosimilar developers with more clarity regarding the intellectual property landscape surrounding reference biologics.37 The disclosure of patents involved in the “patent dance” can offer valuable insights into the potential patent challenges that biosimilar applicants might face, allowing them to make more informed decisions about their development and litigation strategies.9
- Navigating Patent Information and the “Patent Dance”:
The “patent dance” is a patent dispute resolution process established under the BPCIA that involves a series of information exchanges between a biosimilar applicant and the sponsor of the reference product.8 This process is initiated when a biosimilar applicant submits its abbreviated Biologics License Application (aBLA) to the FDA and provides the reference product sponsor with information about its product and manufacturing process.8 Within 60 days, the reference product sponsor must provide the biosimilar applicant with a list of patents that it believes could reasonably be asserted if the biosimilar were to be marketed.8 Subsequent exchanges involve the biosimilar applicant providing its views on non-infringement or invalidity of these patents, followed by the reference product sponsor’s response.8
The Purple Book now includes patent lists that are disclosed during this “patent dance”.41 However, it is crucial to understand that the patent information available in the Purple Book might not be as comprehensive as that found in the Orange Book.37 The Purple Book primarily lists patents that the reference product sponsor has asserted against a specific biosimilar applicant during the “patent dance.” This means that other patents owned or licensed by the reference product sponsor that cover the product but were not part of this specific exchange might not be listed in the Purple Book.9 Furthermore, unlike the Orange Book, there is no ongoing obligation for reference product sponsors to continuously update the patent information in the Purple Book beyond the initial disclosures made during the patent dance.37 Therefore, biosimilar developers should not solely rely on the Purple Book for a complete understanding of the potential patent challenges they might face.
- Expert Strategies for Patent Research Using the Purple Book:
To effectively conduct patent research using the Purple Book, several expert strategies should be considered. Begin by searching the database using either the proprietary name or the proper name of the biological product. This will provide access to information on the licensed reference product and any associated biosimilars.42 Once a specific product is selected, check the “Product Details” page for an indication of whether a patent list has been provided. This is typically indicated by a “Yes” link under the heading “Patent List Provided”.41 If a patent list is available, carefully analyze the patent numbers and their corresponding expiration dates.41
Pay close attention to the reference product exclusivity expiration dates listed in the Purple Book. These dates provide an estimate of when biosimilar competition might become possible.42 Utilize the advanced search function within the Purple Book database to conduct more targeted searches using various criteria such as applicant name or BLA number.42 The FDA also provides monthly reports containing all the information in the Purple Book, which can be downloaded in various formats for more detailed analysis.43 Given that the patent information in the Purple Book may not be exhaustive, it is essential to cross-reference this information with litigation dockets related to biosimilar approvals. This can help identify patents that have been asserted in court but may not be listed in the Purple Book.9 Finally, for information regarding orphan drug exclusivity, which is not included in the Purple Book, researchers should consult the FDA’s orphan drug database.44 By employing these strategies and recognizing the specific nuances of the Purple Book, researchers can maximize its utility for patent research related to biological products.
IV. Orange Book vs. Purple Book: A Comparative Analysis for Patent Research
- Key Differences in Scope, Content, and Patent Information:
The FDA Orange Book and Purple Book serve distinct yet complementary roles in providing information about approved pharmaceutical products and their intellectual property protections. The Orange Book primarily focuses on small-molecule drugs, offering comprehensive patent information that includes details on drug substance, drug product, and method of use patents.1 A key feature of the Orange Book is the inclusion of patent use codes, which specify the approved uses covered by a patent.6 It also provides detailed information on various regulatory exclusivities, such as NCE, ODE, and pediatric exclusivity.6 In contrast, the Purple Book is dedicated to biological products, and its patent information is less comprehensive.2 It primarily lists patents that have been disclosed during the “patent dance” process between a biosimilar applicant and the reference product sponsor, focusing on patent numbers and expiration dates.41 While the Purple Book does provide information on reference product exclusivity and biosimilarity or interchangeability evaluations, it does not include patent use codes or orphan exclusivity data.40
The patent listing requirements also differ significantly between the two resources. In the Orange Book, NDA applicants are required to proactively list all relevant patents that could be infringed by a generic product.12 However, in the Purple Book, BLA holders are only obligated to list patents if and when they are included in a “patent list” during the “patent dance” stage of biosimilar litigation under the BPCIA.10 Another notable difference lies in the treatment of manufacturing patents. The Orange Book generally excludes patents covering manufacturing processes.21 However, the Purple Book could potentially include method of manufacture patents if these are raised by the reference product sponsor against a specific biosimilar applicant during the patent dance.10
| Feature | FDA Orange Book | FDA Purple Book |
| Drugs Covered | Small-molecule drugs | Biological products (large-molecule drugs) |
| Patent Information | Comprehensive: drug substance, drug product, method of use patents; patent use codes; submission and expiration dates | Less comprehensive: primarily patents disclosed during the “patent dance”; patent number and expiration date |
| Exclusivity Info | Detailed: NCE, ODE, pediatric, GAIN, 180-day, CGT, etc. | Focus on reference product exclusivity and biosimilar/interchangeable exclusivity; does not include orphan exclusivity |
| Patent Use Codes | Yes | No |
| Update Frequency | Daily (generic approvals), monthly (supplements), annually (edition) | Monthly |
| Patent Listing Trigger | NDA approval | Disclosure during the “patent dance” (triggered by biosimilar application) |
| Manufacturing Patents | Generally excluded | May be included if raised during the patent dance |
| Legal Framework | Hatch-Waxman Act | Biologics Price Competition and Innovation Act (BPCIA) |
| Comprehensiveness | Generally more comprehensive for listed patent types | Less comprehensive; requires supplementation with other resources |
The fundamental distinction between the Orange and Purple Books stems from the different regulatory frameworks governing small-molecule drugs and biologics. The more comprehensive patent disclosure in the Orange Book provides generic drug developers with greater certainty and facilitates more proactive patent challenges compared to the Purple Book’s more limited disclosure for biosimilars.2 This historical disparity has often been cited as a contributing factor to the challenges faced by biosimilar manufacturers in navigating the patent landscape.
- Strengths and Limitations of Each Resource for Different Research Needs:
Both the Orange and Purple Books offer unique strengths and limitations for pharmaceutical patent research. The Orange Book’s strengths include its comprehensive patent information for small-molecule drugs, the valuable inclusion of patent use codes that aid in understanding the scope of method-of-use patents, and its detailed coverage of various regulatory exclusivities.3 Furthermore, the Orange Book benefits from a long-established legal and regulatory precedent under the Hatch-Waxman Act.6 However, its limitations include the general exclusion of manufacturing process patents and the fact that it may not always immediately reflect the outcomes of recent patent litigation.23
The Purple Book’s strengths lie in its role as a centralized source for information on licensed biological products, its provision of reference product exclusivity data, and its evaluations of biosimilarity and interchangeability.5 The recent legislative mandates have also begun to increase patent transparency within the Purple Book.38 However, its limitations include the fact that patent information is less comprehensive and contingent on the “patent dance” process.9 It also lacks patent use codes, which are crucial for understanding the nuances of method-of-use patents, and it does not include data on orphan drug exclusivity.40
Researchers need to consider these strengths and limitations when deciding which resource is most appropriate for their specific research needs. The Orange Book is generally more suitable for in-depth patent analysis of small-molecule drugs, particularly when understanding patent use codes is critical. The Purple Book, on the other hand, is the primary resource for obtaining information on biological products, reference product exclusivity, and the status of biosimilar development. Given the inherent limitations of each book, a comprehensive patent research strategy often necessitates consulting both resources, depending on the drugs being investigated, as well as supplementing this information with other external patent databases and legal resources.
- Strategic Considerations for Choosing the Right Resource:
When embarking on pharmaceutical patent research using FDA resources, the first strategic consideration is to identify whether the drug of interest is a small molecule or a biologic.1 Small-molecule drugs are typically listed in the Orange Book, while biological products are found in the Purple Book.44 Once the type of drug is determined, the next step is to consider the specific type of information that is needed. For instance, if the research focus is on understanding the expiration dates of patents covering a small-molecule drug or identifying potential patent carve-out strategies for a generic, the Orange Book would be the more appropriate primary resource. Conversely, if the goal is to determine the reference product exclusivity period for a biologic or to identify any patents that have been disclosed during the “patent dance” for a biosimilar, the Purple Book should be consulted first.
It is also important to consider the stage of drug development or market entry being researched. For generic drug development related to small molecules, the Orange Book is indispensable for navigating the Hatch-Waxman framework. For biosimilar development, the Purple Book provides crucial information about reference product exclusivity and the evolving patent landscape under the BPCIA. Finally, researchers must remain cognizant of the limitations of each book. The Orange Book may not provide a complete picture of all relevant patents, especially those related to manufacturing, while the Purple Book’s patent information is still developing and may require supplementation. Therefore, a holistic approach that involves utilizing both resources when applicable and supplementing with other patent databases and legal resources is often the most effective strategy for comprehensive drug patent research.
V. Advanced Tips and Best Practices for Comprehensive Drug Patent Research
- Integrating Insights from Both the Orange and Purple Books:
For pharmaceutical companies that have a diverse portfolio encompassing both small-molecule drugs and biological products, gaining insights from both the Orange and Purple Books is essential for a comprehensive understanding of the competitive landscape. By tracking patent and exclusivity information for all related products across both resources, companies can develop a broader market perspective and make more informed strategic decisions regarding research and development, intellectual property management, and market access. This integrated approach allows for a more holistic view of potential future competition and opportunities in both the generic and biosimilar markets.
- Mastering Patent Use Codes and Exclusivity Codes for Strategic Advantage:
A thorough understanding of patent use codes in the Orange Book can provide a significant strategic advantage, particularly for generic drug developers. By utilizing the “(https://www.accessdata.fda.gov/scripts/cder/ob/results_patent.cfm)”, researchers can gain a detailed understanding of the specific aspects of a small-molecule drug that are protected by method-of-use patents. This granular level of analysis is crucial for formulating effective “skinny label” strategies aimed at carving out patented uses and achieving earlier generic market entry. Similarly, mastering the various exclusivity codes and their definitions, available through the “(https://www.accessdata.fda.gov/scripts/cder/ob/results_exclusivity.cfm)”, is vital for strategic planning. Knowledge of the different types and durations of exclusivities allows companies to accurately forecast market competition and develop robust product lifecycle management strategies. It is important to note that the Purple Book currently does not utilize patent use codes in the same manner as the Orange Book, reflecting the different regulatory pathways for biologics.
- Staying Abreast of Updates, Guidance, and Legislative Changes:
The pharmaceutical regulatory and intellectual property landscape is constantly evolving. Therefore, it is imperative for researchers to stay informed about the latest updates to both the Orange and Purple Books. Regularly checking the FDA websites for new monthly supplements, annual editions, and any newly issued guidance documents or Federal Register notices is crucial for maintaining accurate and current knowledge.3 Additionally, it is important to monitor any legislative changes that may impact patent listing requirements and transparency within these resources, such as the Orange Book Transparency Act and the Purple Book Continuity Act.10 Staying abreast of these changes ensures that research is based on the most up-to-date information, which is essential for making sound strategic decisions.
- Understanding the Legal and Regulatory Framework Governing Patent Listings:
To effectively utilize the Orange and Purple Books for patent research, a solid understanding of the underlying legal and regulatory frameworks is essential. Familiarity with the Hatch-Waxman Act, which governs generic drug approvals and patent listings for small molecules, and the BPCIA, which provides the framework for biosimilar approvals and the Purple Book, is crucial.6 Understanding the significance of Paragraph IV certifications in the context of Orange Book patent listings, including the potential for a 30-month stay on generic approval, is also vital for generic drug developers.12 For research involving biologics, a thorough understanding of the “patent dance” process under the BPCIA and its implications for patent disclosure in the Purple Book is necessary.8 Interpreting the information contained within the Orange and Purple Books requires placing it within this broader legal and regulatory context to fully grasp its implications.
VI. Conclusion: Empowering Pharmaceutical Patent Research with FDA Resources
The FDA Orange and Purple Books stand as indispensable resources for conducting thorough drug patent and exclusivity research within the pharmaceutical industry. The Orange Book provides a wealth of detailed information on small-molecule drugs, including comprehensive patent listings, patent use codes, and various regulatory exclusivities. It serves as the cornerstone for navigating the generic drug approval pathway established by the Hatch-Waxman Act. The Purple Book, while still evolving in terms of patent transparency, offers crucial insights into the world of biological products, detailing reference product exclusivity and the status of biosimilar development under the BPCIA.
Effective utilization of these FDA resources necessitates a comprehensive approach. This involves not only understanding the specific content of each book but also recognizing their individual strengths and limitations. Researchers must often integrate information from both the Orange and Purple Books, particularly when dealing with companies that have a diverse product portfolio. Furthermore, a thorough understanding of the relevant legal and regulatory landscape, including the nuances of patent use codes, exclusivity provisions, and the patent dispute resolution processes outlined in the Hatch-Waxman Act and the BPCIA, is paramount for accurate interpretation and strategic decision-making. By mastering the intricacies of the Orange and Purple Books and staying informed about ongoing updates and legislative changes, pharmaceutical professionals and legal experts can significantly enhance their patent research capabilities and navigate the complex landscape of pharmaceutical intellectual property with greater confidence and strategic advantage.
| Exclusivity Code | Description | Duration |
| NCE | New Chemical Entity – Drug contains no active moiety that has been approved by FDA in any other application under section 505(b) of the FD&C Act. | 5 years |
| ODE | Orphan Drug Exclusivity – Granted for drugs intended to treat rare diseases or conditions. | 7 years |
| NCS | New Clinical Study – Exclusivity based on new clinical investigations (other than bioavailability studies) essential to approval and conducted or sponsored by the applicant. | 3 years |
| PED | Pediatric Exclusivity – 6 months added to existing patents and/or exclusivity for conducting pediatric studies in response to an FDA written request. | 6 months (added to existing patent/exclusivity) |
| GAIN | Generating Antibiotic Incentives Now – Additional 5 years of exclusivity added to certain other exclusivity periods for a drug product granted a Qualified Infectious Disease Product designation. | 5 years (added to NCE, ODE, NCS) |
| PC | Patent Challenge (180-day Exclusivity) – May be granted to the first generic applicant to submit a substantially complete ANDA containing a challenge to a patent listed in the Orange Book. | 180 days |
| CGT | Competitive Generic Therapy – 180 days of exclusivity for applications for drug products with inadequate generic competition. | 180 days |
| Other (e.g., NP) | Various other codes may indicate exclusivity for new products, new dosage forms, new patient populations, etc., often tied to the 3-year New Clinical Study exclusivity if supported by new clinical investigations. The specific definitions can be found in the FDA resources. | Typically 3 years, depending on the specific code and the basis for the exclusivity (often linked to new clinical studies). Refer to the “(https://www.accessdata.fda.gov/scripts/cder/ob/results_exclusivity.cfm)”. |
Works cited
- Patent Listing in FDA’s Orange Book – Congress.gov, accessed May 15, 2025, https://www.congress.gov/crs-product/IF12644
- Paucity of intellectual property rights information in the US biologics system a decade after passage of the Biosimilars Act – PMC, accessed May 15, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC11081489/
- Approved Drug Products with Therapeutic Equivalence Evaluations | Orange Book – FDA, accessed May 15, 2025, https://www.fda.gov/drugs/drug-approvals-and-databases/approved-drug-products-therapeutic-equivalence-evaluations-orange-book
- Patent Listing in FDA’s Orange Book, accessed May 15, 2025, https://sgp.fas.org/crs/misc/IF12644.pdf
- www.fda.gov, accessed May 15, 2025, https://www.fda.gov/media/90150/download
- Orange Book 101 | The FDA’s Official Register of Drugs, accessed May 15, 2025, https://www.fr.com/insights/ip-law-essentials/orange-book-101/
- Hatch-Waxman 101 – Fish & Richardson, accessed May 15, 2025, https://www.fr.com/insights/thought-leadership/blogs/hatch-waxman-101-3/
- Taking Advantage of the New Purple Book Patent Requirements for Biologics, accessed May 15, 2025, https://www.morganlewis.com/pubs/2021/04/taking-advantage-of-the-new-purple-book-patent-requirements-for-biologics
- Opinion: Purple Book Patent Listings Are Only a First Step – Center for Biosimilars®, accessed May 15, 2025, https://www.centerforbiosimilars.com/view/opinion-purple-book-patent-listings-are-only-a-first-step
- New Orange Book And Purple Book Patent Listing Laws Impose New Requirements, accessed May 15, 2025, https://www.foley.com/insights/publications/2021/03/new-orange-book-purple-book-patent-listing-laws/
- New Orange & Purple Book laws increase transparency of patent information for drugs, biologics – Hogan Lovells, accessed May 15, 2025, https://www.hoganlovells.com/en/publications/new-orange-purple-book-laws-increase-transparency-of-patent-information-for-drugs-biologics
- Patents and Exclusivities for Generic Drug Products – FDA, accessed May 15, 2025, https://www.fda.gov/drugs/cder-conversations/patents-and-exclusivities-generic-drug-products
- The Purple Book: A Compendium of Biological and Biosimilar Products – U.S. Pharmacist, accessed May 15, 2025, https://www.uspharmacist.com/article/the-purple-book-a-compendium-of-biological-and-biosimilar-products
- Drug Patent and Exclusivity Study available – USPTO, accessed May 15, 2025, https://www.uspto.gov/initiatives/fda-collaboration/drug-patent-and-exclusivity-study-available
- The Purple Book Demystified: What Biopharma Companies Need to …, accessed May 15, 2025, https://patentskart.com/the-purple-book-demystified-what-biopharma-companies-need-to-know-about-patent-expirations-and-biosimilars/
- Orange Book Questions and Answers – FDA, accessed May 15, 2025, https://www.fda.gov/media/160167/download
- Orange Book Preface – FDA, accessed May 15, 2025, https://www.fda.gov/drugs/development-approval-process-drugs/orange-book-preface
- Orange Book 101: An Overview of FDA’s Orange Book – YouTube, accessed May 15, 2025, https://www.youtube.com/watch?v=DYYWZxfWB9o
- Resources Available on the Orange Book Website – FDA, accessed May 15, 2025, https://www.fda.gov/media/168938/download
- Electronic Orange Book – FDA, accessed May 15, 2025, https://www.fda.gov/drugs/fda-drug-info-rounds-video/electronic-orange-book
- The Listing of Patent Information in the Orange Book – FDA, accessed May 15, 2025, https://www.fda.gov/media/155200/download
- www.mayerbrown.com, accessed May 15, 2025, https://www.mayerbrown.com/-/media/files/perspectives-events/events/2023/06/mayer-brown–fda-lifecycle-management-webinar-patent-listing.pdf%3Frev=-1
- Patents and Exclusivity | FDA, accessed May 15, 2025, https://www.fda.gov/media/92548/download
- Patent Use Codes for Pharmaceutical Products: A Comprehensive Analysis, accessed May 15, 2025, https://www.drugpatentwatch.com/blog/patent-use-codes-for-pharmaceutical-products-a-comprehensive-analysis/
- Expanding the Scope of the Hatch-Waxman Act’s Patent Carve-out Exception to the Identical Drug Labelling Requirement, accessed May 15, 2025, https://scholarship.law.upenn.edu/context/penn_law_review/article/1216/viewcontent/Dohm156U.Pa.L.Rev.151_282007_29.pdf
- Orange Book Data Files – FDA, accessed May 15, 2025, https://www.fda.gov/drugs/drug-approvals-and-databases/orange-book-data-files
- Revisiting FDA’s Original Guidance on Orange Book Listability in Light of Heightened FTC Scrutiny | Foley & Lardner LLP, accessed May 15, 2025, https://www.foley.com/insights/publications/2023/11/fda-guidance-orange-book-listability-ftc/
- Frequently Asked Questions on Patents and Exclusivity – FDA, accessed May 15, 2025, https://www.fda.gov/drugs/development-approval-process-drugs/frequently-asked-questions-patents-and-exclusivity
- Drug Marketing Exclusivity: Types & Developer Benefits – Allucent, accessed May 15, 2025, https://www.allucent.com/resources/blog/types-marketing-exclusivity-drug-development
- Five (5) & Ten (10) Year Data Exclusivity for New Drugs – When To File Generic Drug Applications – IP & FDA Lawyers, accessed May 15, 2025, https://ipfdalaw.com/five-5-ten-10-year-data-exclusivity-for-new-drugs-when-to-file-generic-drug-applications/
- Three-Year Exclusivity – Mayer Brown, accessed May 15, 2025, https://www.mayerbrown.com/-/media/files/perspectives-events/events/2023/05/fda-lifecycle-management-webinar-3year-new-clinical-investigation-exclusivity–may-11-2023-final.pdf%3Frev=-1
- Orange Book: 101 An Overview (11of27) Generic Drugs Forum 2018 – YouTube, accessed May 15, 2025, https://www.youtube.com/watch?v=Ru1xAPs8iFs
- Exclusivity and Generic Drugs: What Does It Mean? | FDA, accessed May 15, 2025, https://www.fda.gov/files/drugs/published/Exclusivity-and-Generic-Drugs–What-Does-It-Mean-.pdf
- Purple Book: Lists of Licensed Biological Products with Reference Product Exclusivity and Biosimilarity or Interchangeability Evaluations | BBCIC, accessed May 15, 2025, https://www.bbcic.org/resources/fda-guidance/Purple-Book
- Background Information: List of Licensed Biological Products with Reference Product Exclusivity and Biosimilarity or Interchangeability Evaluations (Purple Book) | FDA, accessed May 15, 2025, https://www.fda.gov/drugs/biosimilars/background-information-list-licensed-biological-products-reference-product-exclusivity-and
- Purple Book: Lists of Licensed Biological Products with Reference Product Exclusivity and Biosimilarity or Interchangeability Evaluations | FDA, accessed May 15, 2025, https://www.fda.gov/drugs/therapeutic-biologics-applications-bla/purple-book-lists-licensed-biological-products-reference-product-exclusivity-and-biosimilarity-or
- Revamped Purple Book Offers Greater Patent Transparency for Biologics – Morgan Lewis, accessed May 15, 2025, https://www.morganlewis.com/blogs/asprescribed/2021/05/revamped-purple-book-offers-greater-patent-transparency-for-biologics
- Changes to the Purple Book: Progress in Transparency | Biosimilars Law Bulletin, accessed May 15, 2025, https://www.biosimilarsip.com/2021/08/02/changes-to-the-purple-book-progress-in-transparency/
- www.drugpatentwatch.com, accessed May 15, 2025, https://www.drugpatentwatch.com/blog/drug-patent-research-expert-tips-for-using-the-fda-orange-and-purple-books/#:~:text=Monitor%20Updates%3A%20The%20Orange%20Book,biologics%5B8%5D%5B13%5D.
- The Purple Book and The Orange Book – When do Patents Expire and Regulatory Exclusivities end for FDA Approved Products? | Insights & Resources | Goodwin, accessed May 15, 2025, https://www.goodwinlaw.com/en/insights/blogs/2020/09/the-purple-book-and-the-orange-book–when-do-paten
- Patent Lists – FDA Purple Book, accessed May 15, 2025, https://purplebooksearch.fda.gov/patent-list
- User Guide – FDA Purple Book, accessed May 15, 2025, https://purplebooksearch.fda.gov/userguide
- A Walk-Through of FDA’s Purple Book: Database of Biological Products – YouTube, accessed May 15, 2025, https://www.youtube.com/watch?v=1spYxHhXWck
- What are the FDA’s Orange, Purple, and Green Books? – Katrina Rogers Consulting, accessed May 15, 2025, https://krogersconsulting.com/science/exploring-the-fdas-color-books/
- Pharmaceutical Patent Regulation in the United States – The Actuary Magazine, accessed May 15, 2025, https://www.theactuarymagazine.org/pharmaceutical-patent-regulation-in-the-united-states/