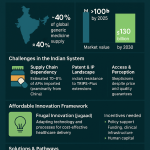
The global healthcare landscape is undergoing a profound transformation, driven significantly by the escalating burden of chronic diseases. These conditions, often referred to as Noncommunicable Diseases (NCDs), encompass a wide array of illnesses such as heart disease, stroke, cancer, diabetes, and chronic lung disease. Alarmingly, these NCDs are collectively responsible for a staggering 74% of all deaths worldwide . This is not merely a public health crisis; it presents a formidable economic and social challenge, particularly evident in low- and middle-income countries, where over three-quarters of all NCD-related deaths occur . The pervasive and growing burden of chronic diseases creates an ever-expanding market for long-term pharmaceutical interventions. Companies engaged in generic drug development are not simply entering a commercial market; they are stepping into a critical public health mission. The widespread and persistent nature of NCDs necessitates continuous management, which translates directly into a sustained, high-volume demand for medications. If these essential medications remain prohibitively expensive, patient access and adherence inevitably suffer, thereby exacerbating the public health crisis. Consequently, the provision of affordable generic options emerges not just as a business opportunity, but as a societal necessity, aligning commercial interests with broader public health goals.
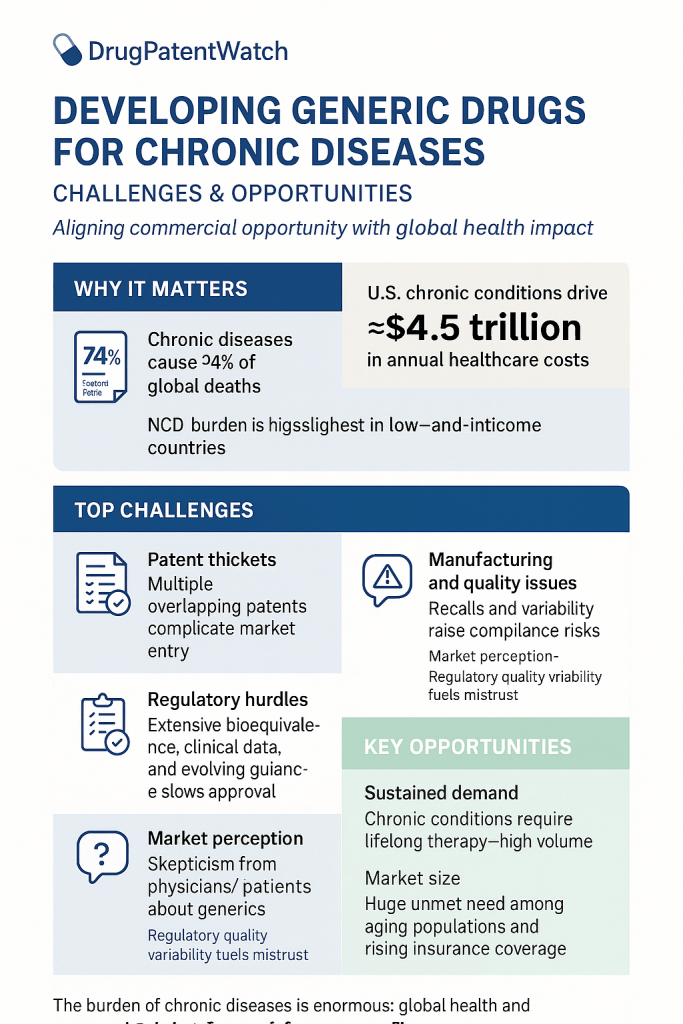
The economic strain imposed by NCDs on healthcare systems globally further amplifies the demand for cost-effective pharmaceutical solutions. In the United States alone, chronic diseases are the leading drivers of an estimated $4.5 trillion in annual healthcare costs. This immense financial pressure compels governments, healthcare payers, and individual patients to actively seek more affordable alternatives for managing long-term conditions. Generic drugs, by their very nature, offer a direct and substantial pathway to this cost reduction, establishing a clear link between the escalating disease burden, healthcare expenditures, and the burgeoning demand for generic medications.
Generic drugs stand as a cornerstone of accessible and affordable healthcare. They are not merely cheaper alternatives; they are scientifically proven copies of brand-name medications. The U.S. Food and Drug Administration (FDA) mandates that generic drugs must contain the same active ingredient, strength, dosage form, and route of administration as their brand-name counterparts . This stringent requirement ensures that the core therapeutic component of the medicine is identical. The FDA rigorously ensures that generic drugs meet the same exacting quality, safety, and efficacy standards as the brand-name products they emulate . This commitment to equivalence means that generic drugs perform approximately the same in the body as their branded versions.
The significantly lower price point of generic medications—often 80-85% cheaper than brand-name equivalents—stems from a fundamental difference in their development pathway . Generic manufacturers are not required to repeat the costly, time-consuming animal and human clinical trials that are essential for the development of new drugs. Furthermore, they generally do not incur the substantial expenses associated with extensive advertising, marketing, and promotion campaigns typical of brand-name products . This inherent cost-effectiveness translates into monumental savings for patients and healthcare systems alike. For instance, in 2016, generic drugs accounted for a remarkable 89% of all prescriptions dispensed in the U.S., yet they represented only 26% of the total drug costs . This disproportionate value underscores that the economic impact of generics is not marginal; it is foundational to healthcare affordability and sustainability, creating a compelling value proposition for both patients and payers. This is not just a minor saving; it represents a systemic cost-containment mechanism that enables healthcare systems to extend their budgets further and allows patients to access necessary treatments. This makes generics an indispensable component of any sustainable healthcare model.
Moreover, the affordability of generic drugs directly enhances medication adherence, which in turn leads to improved health outcomes and reduced overall healthcare costs. High prescription drug costs frequently act as a significant barrier to patients taking their medications as prescribed, with many individuals reporting that they have skipped or postponed needed care and prescriptions due to financial concerns. Generic drugs effectively lower this financial barrier. Studies have demonstrated that patients who are initiated on generic drugs are more likely to adhere to their medication regimens compared to those started on brand-name drugs. Improved adherence directly correlates with better chronic disease management, fewer complications, and ultimately, lower long-term healthcare expenditures, including reduced hospitalizations and emergency visits. This creates a beneficial cycle: increased affordability leads to improved adherence, which results in better health outcomes, and subsequently, significant cost savings for the healthcare system.
Despite their undeniable benefits and critical role, the journey of developing and commercializing generic drugs for chronic diseases is fraught with complexities. These include navigating intricate regulatory pathways, overcoming formidable intellectual property hurdles, ensuring robust manufacturing quality and supply chain resilience, and addressing persistent patient and physician perceptions. However, within these challenges lie immense opportunities for innovative pharmaceutical companies. The evolving landscape, marked by significant patent expirations, rapid technological advancements, and a growing emphasis on value-based care, presents fertile ground for strategic growth in the generic sector, particularly in the burgeoning realms of complex generics and biosimilars. The generic drug industry is evolving beyond simple “copycat” models towards a more sophisticated landscape focused on “higher-value generics” and strategic differentiation. The intense price competition prevalent in the market for “simple” generics inevitably drives down profit margins, making this segment less attractive for sustained investment. This economic pressure compels generic manufacturers to innovate, shifting their focus towards complex generics and biosimilars that offer improved efficacy, enhanced safety, or greater convenience for patients . This strategic pivot is a natural market response to commoditization, signaling a clear move towards a more innovation-driven and value-added generic pharmaceutical sector.
Understanding the Fundamentals: What Exactly is a Generic Drug?
To fully appreciate the strategic landscape of generic drug development, it is essential to establish a foundational understanding of what constitutes a generic drug and how it differs from its brand-name counterpart, as well as from other categories of follow-on medicines like biosimilars and complex generics.
Defining Generic Drugs: Active Ingredients, Bioequivalence, and Therapeutic Equivalence
At its core, a generic drug is designed to be a pharmaceutical equivalent to its brand-name counterpart. The FDA explicitly defines a generic drug as one that must have the “same active ingredient, strength, dosage form, and route of administration as the brand-name drug”. This fundamental requirement ensures that the therapeutic component—the very essence of the medicine responsible for treating the illness or condition—is identical to that of the original product.
The cornerstone of generic drug approval is the demonstration of bioequivalence. This critical standard means that the generic drug must deliver the active ingredient to the bloodstream at approximately the same rate and to the same extent as the brand-name drug, thereby ensuring similar therapeutic effects . To establish this, generic drug companies perform rigorous pharmacokinetic studies, typically involving single-dose comparison studies in healthy volunteers . These studies measure the concentration of the active ingredient in the bloodstream at multiple time points following administration. The FDA generally considers generic drugs bioequivalent if the 90% confidence interval for the ratio of certain pharmacokinetic parameters, specifically the Area Under the Curve (AUC) and maximum concentration (Cmax), falls within an 80% to 125% range of the reference drug values . This statistical benchmark serves as the scientific gatekeeper for generic market entry, providing a quantitative assurance of comparable performance.
While the active ingredients must be identical, it is important to note that minor variations in inactive ingredients (such as fillers, dyes, or preservatives), color, flavor, and packaging are permissible . These differences are allowed as long as they do not affect the way the drug works or its overall performance in the body. This distinction is a key point that is often misunderstood by the public, sometimes leading to unwarranted concerns about generic products.
Despite these rigorous bioequivalence standards and the FDA’s official position that approved generic drugs are equivalent to their branded counterparts , there have been reported patient experiences of “undesired effects” when switching between brand-name drugs and generic formulations, or even between different generic versions . Physicians and patients have, in some instances, reported problems with generic drugs, suggesting that they do not always have the same desired impact as their branded counterparts . The FDA acknowledges these reports and encourages the generic industry to investigate the circumstances under which such problems occur. This highlights a subtle yet significant gap between scientific equivalence, as measured by pharmacokinetic parameters, and real-world patient perception, which can ultimately impact medication adherence. The existence of these reports, even if not medically significant in all cases, implies that while the pharmacokinetic profile may be equivalent, other factors—such as variations in inactive ingredients, subtle manufacturing differences beyond current detection limits, or even psychological effects—might lead to perceived or actual clinical differences for some patients. This perception, regardless of its scientific basis, can undermine patient trust and adherence, posing a critical business challenge for generic manufacturers and the broader healthcare system.
Beyond the Basics: Small Molecule Generics vs. Complex Generics vs. Biosimilars
The term “generic drug” is frequently used as an umbrella term, but the pharmaceutical landscape is far more nuanced. It encompasses distinct categories of follow-on medicines, each with varying levels of molecular complexity, development challenges, and specific regulatory pathways. Understanding these distinctions is paramount for any strategic planning within the pharmaceutical industry.
Small Molecule Generics: The Traditional Pathway
Small molecule generics represent the most common and traditional type of generic drug. These medications are typically synthesized through chemical processes and are characterized by their relatively small and straightforward molecular structures . Common examples include widely used medications such as aspirin, ibuprofen, atorvastatin (Lipitor), amlodipine (Norvasc), and metformin (Glucophage) . Their chemical identity allows for a precise duplication of the active ingredient, making them comparatively simpler to reproduce and to demonstrate bioequivalence to their brand-name counterparts. The approval process for these drugs primarily utilizes the Abbreviated New Drug Application (ANDA) pathway, which, by design, does not require the generic drug company to repeat the costly animal and clinical research already conducted for the original brand-name drug .
The chemical simplicity of small molecule drugs directly leads to lower development costs and faster market entry for generics. This, in turn, drives rapid and significant price erosion once multiple competitors emerge. The straightforward nature of their chemical structures means that synthesis and characterization are less complex, substantially reducing research and development (R&D) expenditures. Developing a small molecule generic can cost significantly less, typically ranging from $1 million to $4 million. This relatively low barrier to entry attracts numerous generic manufacturers, fostering intense competition in the market. This competition is a direct consequence of the ease of replication and the lower development cost. For instance, the entry of multiple generic companies can lead to price reductions of up to 95% when six or more competitors are present in the market. This economic dynamic underscores the need for generic manufacturers to prioritize efficiency and speed-to-market in this segment.
Biosimilars: The Biologic Counterparts
Biosimilars are a distinct category of follow-on medicines, representing versions of brand-name biological products. Unlike small molecule drugs, biological medicines are large, complex molecules that are typically manufactured from living systems, such as microorganisms (e.g., yeast, bacteria) or animal cells . Examples include vaccines, blood components, gene therapies, and complex therapeutic proteins. Due to the inherent natural variability of biological manufacturing processes, biosimilars are defined as “highly similar” to their reference product, rather than identical . This subtle but crucial distinction reflects the scientific reality that an exact replication of a complex biological molecule, with all its post-translational modifications and micro-heterogeneities, is not feasible.
Biosimilars are approved through a distinct abbreviated pathway in the U.S., specifically the 351(k) pathway under the Biologics Price Competition and Innovation Act (BPCIA), which differs from the ANDA pathway for small molecules. While this pathway avoids duplicating all the costly clinical trials required for a novel biologic, it still necessitates comprehensive comparability studies . These studies typically involve extensive analytical characterization to demonstrate structural and functional similarity, as well as animal studies and at least one comparative clinical study to confirm that there are no clinically meaningful differences between the biosimilar and the reference product in terms of safety, purity, and potency . Developing a biosimilar is a significantly more complex and costly undertaking than developing a small molecule generic, often requiring 7 to 8 years and an investment exceeding $100 million.
The “highly similar” nature and higher development cost of biosimilars create a market dynamic that is notably distinct from that of small-molecule generics. This segment offers the potential for higher margins but demands greater scientific and regulatory expertise. Because biosimilars cannot be “identical” and require more extensive and sophisticated testing than small-molecule generics, their development cost is substantially higher. This elevated barrier to entry naturally limits the number of competitors compared to the simple generic market, allowing for more sustainable profit margins. For instance, the price reduction for biosimilars compared to their reference biologics typically ranges from 15-30%, in contrast to the 80% or more seen with small-molecule generics. This implies that biosimilar development requires a different investment profile and a more sophisticated scientific approach, but also promises a more sustainable competitive landscape with less aggressive price erosion.
Complex Generics: A Spectrum of Innovation
The category of “complex generics” encompasses a broad and diverse group of pharmaceutical products that present unique challenges in their development and approval. While there is no single, universally agreed-upon definition, the FDA generally identifies them as medicines that possess complex active ingredients, formulations, dosage forms, routes of administration, or are classified as complex drug-device combination products . This wide-ranging category includes products such as transdermal patches, liposomal formulations, nanoparticles, and long-acting injectables .
The inherent complexity of these products arises from several factors. It can be challenging to demonstrate bioequivalence, particularly when the drug’s activity is not easily measured in the bloodstream or when it acts locally on a specific organ. Furthermore, complex generics often present significant hurdles related to manufacturing scale-up, the intricacies of the production process itself, and, frequently, a lack of clear and well-defined regulatory pathways . These factors collectively mean that creating and manufacturing complex generics demands higher levels of expertise, meticulous planning, and greater development costs compared to standard, small-molecule generics.
Complex generics represent a frontier where unmet medical needs intersect with significant technical and regulatory hurdles, offering substantial market differentiation and higher value for companies that can navigate these complexities. The inherent difficulty in developing these products means that fewer companies are able to successfully enter this space, leading to less market competition compared to the crowded simple generic market . This creates a “blue ocean” opportunity for pharmaceutical firms capable of overcoming the formidable technical and regulatory challenges. By successfully solving these complex problems, companies can develop what are often termed “higher-value generics” . These products offer enhanced patient benefits, such as reduced dosing frequency or improved safety profiles, and can command better pricing, allowing developers to move beyond the intense price-driven competition of simple generics.
| Feature | Small Molecule Generics | Biosimilars | Complex Generics |
| Active Ingredient Nature | Chemically synthesized | Derived from living systems (e.g., cells, microorganisms) | Chemically synthesized, but with complex structure or delivery |
| Molecular Structure | Small, simple, well-defined | Large, complex, inherent natural variability | Variable, can be small or large, but with complex formulation/delivery |
| Bioequivalence Standard | Identical active ingredient; bioequivalent (same rate & extent of absorption) | “Highly similar” to reference product; no clinically meaningful differences in safety, purity, potency | Bioequivalent, but often requires specific, non-traditional studies to demonstrate equivalence |
| Regulatory Pathway | Abbreviated New Drug Application (ANDA) | Biologics Price Competition and Innovation Act (BPCIA) 351(k) pathway | Primarily ANDA, but often requires additional studies/guidance (can also use 505(b)(2)) |
| Need for Clinical Trials | Generally not required (bioequivalence studies instead) | Targeted clinical studies required (comparability studies, immunogenicity) | May require additional clinical studies beyond standard bioequivalence (e.g., adhesion for patches) |
| Typical Development Cost | Relatively low ($1-4 million) | High (>$100 million) | Moderate to High (more than simple generics, less than biosimilars) |
| Typical Development Timeline | Shorter (e.g., 36 months pre-filing) | Longer (7-8 years) | Longer than simple generics due to complexity and regulatory ambiguity |
| Key Characteristics | Identical active ingredient, simple to reproduce, high competition, significant price erosion | “Similar but not identical,” complex manufacturing, higher margins, less competition than simple generics | Complex active ingredients, formulations, dosage forms, routes of administration, or drug-device combinations; often address unmet needs |
| Market Dynamics | High volume, low margin, intense price competition | Growing market, moderate competition, moderate price reduction (15-30%) | Emerging market, less competition, potential for higher value/margins |
Table 1: Comparison of Follow-On Drug Types
The Regulatory Labyrinth: Pathways to Market Approval
Navigating the regulatory landscape is perhaps the most critical challenge and opportunity for generic drug developers. The pathways to market approval are intricate, demanding rigorous scientific demonstration and meticulous adherence to legal frameworks. Understanding these pathways is fundamental to strategic planning and successful commercialization.
The Abbreviated New Drug Application (ANDA) Pathway in the U.S.
The Abbreviated New Drug Application (ANDA) process is the primary regulatory pathway for generic drug approval in the United States. This streamlined approach was a cornerstone of the landmark Drug Price Competition and Patent Term Restoration Act of 1984, commonly known as the Hatch-Waxman Act . The “abbreviated” nature of the ANDA process is its defining characteristic: it allows generic manufacturers to gain approval without needing to repeat the extensive and costly animal and human clinical trials for safety and effectiveness that were originally required for the brand-name drug . This efficiency significantly reduces the time and financial investment needed to bring a generic product to market.
Bioequivalence Studies: The Scientific Mandate
At the very heart of an ANDA submission lies the scientific mandate to demonstrate bioequivalence to an already approved Reference Listed Drug (RLD) . This is not a mere formality but a rigorous scientific exercise. It typically involves conducting single-dose comparison studies in healthy volunteers, where blood levels of the active ingredient are measured at multiple time points following administration of both the generic and the RLD . The objective is to prove that the generic drug delivers the same amount of active ingredient to the bloodstream at approximately the same rate as the brand-name drug, thereby ensuring comparable therapeutic effects .
The FDA generally considers generic drugs bioequivalent if the 90% confidence interval for the ratio of certain pharmacokinetic parameters—specifically, the Area Under the Curve (AUC), which represents the total amount of drug absorbed, and the maximum concentration (Cmax), which indicates the peak drug level—falls within an 80% to 125% range of the reference drug values . These statistical benchmarks are the scientific gatekeepers for generic entry. Bioequivalence studies are not just a regulatory hurdle; they are the scientific bedrock validating the generic’s therapeutic equivalence, directly influencing physician confidence and patient acceptance. The FDA’s stringent bioequivalence requirements are meticulously designed to ensure that a generic drug will perform in the body identically to its brand-name counterpart. This robust scientific validation, when effectively communicated to healthcare professionals and patients, can foster trust, which is crucial for widespread adoption and adherence, particularly for chronic conditions where consistent drug performance is paramount.
Manufacturing and Quality Control: Ensuring Consistency and Safety
Beyond demonstrating bioequivalence, an ANDA submission must provide exhaustive details on the drug’s formulation, its manufacturing process, and the comprehensive quality control measures in place. Generic manufacturers are strictly required to adhere to Current Good Manufacturing Practices (cGMP) regulations. These regulations establish the minimum requirements for the methods, facilities, and controls employed in the manufacturing, processing, and packing of a drug product. Their purpose is to ensure that every product is safe for use, contains the ingredients and strength it claims to have, and is consistently produced with the required quality .
The FDA conducts thorough reviews of these manufacturing procedures and performs on-site inspections of generic drug manufacturing facilities. These inspections are critical to verify that the manufacturer possesses the necessary facilities, equipment, and capabilities to consistently produce the drug it intends to market . This oversight also includes a requirement to demonstrate that the drug remains stable and does not break down over time, necessitating months-long “stability tests,” and that the container in which the drug is shipped and sold is appropriate for maintaining its quality.
The intense price competition inherent in the generic market, while undeniably beneficial for affordability, paradoxically creates pressures that can compromise manufacturing quality and supply chain resilience. Generic manufacturers often operate on razor-thin profit margins . This economic reality can disincentivize necessary investments in robust, resilient manufacturing processes and advanced quality control systems . The consequence of such underinvestment is a supply chain that becomes inherently prone to disruptions, quality issues, and drug shortages . This directly impacts patient access to essential medications for chronic diseases, highlighting a critical tension between the drive for cost-driven competition and the imperative for supply reliability. In such a commoditized market, cGMP compliance and a robust quality reputation are not merely regulatory necessities but critical competitive differentiators, directly influencing market share and mitigating the risks of costly recalls. Companies that consistently demonstrate superior cGMP compliance and unwavering quality assurance build trust with healthcare providers and payers. This established reputation can translate into preferred formulary placement and higher prescribing rates, offering a significant competitive edge beyond just price. Conversely, any lapse in quality can lead to severe reputational damage, product recalls, and substantial financial losses .
Labeling Requirements and Permissible Differences
The labeling information for a generic drug, including its indications for use, dosage forms, strength, and route of administration, generally must be identical to that of the Reference Listed Drug (RLD) . However, the regulatory framework allows for certain “permissible differences,” particularly concerning patents and exclusivities that may still apply to the brand-name product.
A notable provision within the Hatch-Waxman Act is the “skinny-label” mechanism. This allows a generic manufacturer to seek FDA approval for only those approved uses of the drug that are no longer protected by patents, while omitting references to still-patented uses from their generic label . This requires meticulous navigation to ensure that the removal of information does not compromise the safe use of the drug. “Skinny-labeling” is a nuanced legal strategy that enables earlier generic market entry but can expose generic manufacturers to complex patent infringement litigation, requiring sophisticated legal and patent intelligence. The ability to “carve out” patented indications allows generic companies to potentially enter the market faster, bypassing certain patent protections. However, this is far from a simple process. It demands meticulous analysis of patent claims and use codes. As demonstrated in cases such as GSK v. Teva, generic manufacturers can still face active inducement of infringement claims, even with carefully carved-out labels. This underscores the critical need for generic companies to possess robust legal counsel and to leverage advanced tools, such as those offered by DrugPatentWatch, to effectively navigate this complex intellectual property landscape.
The Hatch-Waxman Act of 1984: A Landmark Legislation
The Drug Price Competition and Patent Term Restoration Act of 1984, widely known as the Hatch-Waxman Act, stands as a landmark piece of legislation that fundamentally reshaped the pharmaceutical industry. Its core purpose was to strike a delicate balance: to increase the availability of affordable generic drugs while simultaneously preserving the incentives for pharmaceutical companies to innovate and develop new medicines.
This act achieved its objectives by establishing the ANDA pathway, which streamlined generic approvals. It also introduced a system of patent certifications (Paragraph I, II, III, and IV), allowing generic companies to challenge existing patents. Crucially, the Act provided a powerful incentive for generic entry: the concept of 180-day exclusivity for the first generic applicant to successfully challenge a listed patent . This legislation forms the bedrock of the modern generic drug industry, influencing market dynamics and competitive strategies to this day. The Hatch-Waxman Act, while over three decades old, continues to be the primary driver of generic competition and affordability. However, its very mechanisms, such as Paragraph IV challenges and the associated 180-day exclusivity, also create intense legal and market dynamics that generic companies must master. The Act’s dual purpose—promoting generics while protecting innovation—has led to a highly competitive environment. The incentives it created, particularly the 180-day exclusivity, have made Paragraph IV challenges a “routine part of doing business” for generic companies. This means that while the Act effectively delivers on its promise of lower prices, it also necessitates significant legal and strategic investment from generic firms to fully capitalize on its provisions.
The 505(b)(2) New Drug Application: A Hybrid Approach for Innovation
Beyond the traditional ANDA pathway, the 505(b)(2) New Drug Application (NDA) offers a unique and appealing regulatory strategy for pharmaceutical companies. Often referred to as a “hybrid application,” this pathway incorporates elements of both a full New Drug Application (NDA), typically used for novel chemical entities, and an Abbreviated New Drug Application (ANDA). It allows an applicant to rely, in part, on the FDA’s previous findings of safety and efficacy for a previously approved drug, or on data available in the public domain, without needing to conduct all the original clinical trials .
Leveraging Existing Data: Efficiency and Reduced Risk
The primary benefit of the 505(b)(2) pathway lies in its inherent efficiency. By leveraging existing data, it significantly reduces the need for extensive nonclinical and clinical studies that would otherwise be required for a traditional 505(b)(1) NDA . This streamlined approach translates directly into lower development costs and accelerated timelines for market entry. The ability to “bridge” known information about a previously approved active ingredient to a novel drug product or indication minimizes development risk, making it an attractive route for strategic product development.
The 505(b)(2) pathway is a strategic sweet spot for generic companies seeking to differentiate themselves and alleviate competitive pressures by developing “branded generics” or improved versions of existing drugs. In a market segment that is increasingly commoditized by simple generics, the 505(b)(2) pathway offers a distinct route to create new, differentiated products that can secure their own market exclusivity. This enables generic companies to move beyond pure price competition and capture additional value by offering improved formulations, new indications, or more convenient dosing regimens. Such differentiated products can appeal to both patients and prescribers who might otherwise be hesitant about “standard” generics, thereby enhancing market acceptance and profitability.
Ideal Candidates and Market Exclusivity Potential
Ideal candidates for the 505(b)(2) pathway are diverse and include drugs with new indications, changes in dosage form, strength, formulation, dosing regimen, or route of administration. This pathway is also suitable for new combination products or prodrugs of an existing drug . The flexibility of this pathway allows for various types of modifications that can add significant value to an existing active ingredient.
Crucially, products approved under the 505(b)(2) pathway can qualify for various types of market exclusivity, providing a period of protection from generic competition. These exclusivities are statutory and include: Orphan Drug Exclusivity (7 years for drugs treating rare diseases), New Chemical Entity (NCE) Exclusivity (5 years for a drug with no previously approved active moiety), and “Other” Exclusivity (3 years for a change to an already approved drug, such as a new way of delivering the active ingredient or a different disease indication, provided new clinical studies were conducted to support the change) . This potential for market exclusivity transforms a “generic” development effort into a high-value asset, justifying greater R&D investment and offering a more predictable return than pure generic plays. Unlike standard generics that often face immediate and steep price erosion upon market entry, 505(b)(2) products can secure periods of market exclusivity. This exclusivity acts as a protective shield, allowing companies to recoup their investment and generate substantial revenue before intense generic competition eventually emerges. This makes the 505(b)(2) pathway particularly attractive for companies aiming to build a sustainable portfolio of differentiated pharmaceutical products.
The Biosimilar Approval Pathway (351(k))
Biosimilars, as biological medicines, follow a distinct and rigorous regulatory pathway established under the Biologics Price Competition and Innovation Act (BPCIA) in the U.S., specifically the 351(k) pathway. This act, while distinct, shares many similarities with the Hatch-Waxman Act for small molecules, aiming to foster competition in the biologics market. The European Medicines Agency (EMA) has been a pioneer in biosimilar regulation, having approved its first biosimilar as early as 2006, establishing a solid framework that has influenced global development.
Demonstrating “Highly Similar”: Analytical, Animal, and Clinical Comparability
The core principle for biosimilar approval is demonstrating that the product is “highly similar” to a U.S.-licensed reference biological product, and that there are “no clinically meaningful differences” between the biosimilar and the reference product in terms of safety, purity, and potency . This standard is inherently more complex than the “bioequivalence” required for small molecules, primarily due to the intricate nature and inherent natural variability of biological products that are derived from living systems .
The approval process for biosimilars requires a comprehensive “totality of the evidence” approach. This includes extensive analytical characterization to demonstrate structural and functional similarity, often involving sophisticated laboratory techniques . In addition, animal studies and at least one comparative clinical study are typically required to assess potential differences in immunogenicity (the propensity to induce an immune response), pharmacokinetics (PK), pharmacodynamics (PD), efficacy, and safety . This rigorous scientific program is designed to avoid unnecessary repetition of large-scale, costly clinical trials that were performed for the original reference biologic, yet it remains a demanding and expensive endeavor, often taking 7 to 8 years and costing over $100 million . The “highly similar” standard for biosimilars, while abbreviated compared to a de novo biologic, demands a deeper scientific understanding and more sophisticated analytical capabilities than small-molecule generic development, reflecting the inherent complexity of biologics. Biological products, being complex, large molecules with natural variability, cannot be proven “identical” in the same way as small molecules. Therefore, the “highly similar” standard necessitates the use of advanced analytical techniques to meticulously compare structural and functional attributes. Furthermore, targeted clinical studies are crucial to definitively rule out any clinically meaningful differences. This elevates the scientific bar for biosimilar developers, requiring significant investment in specialized R&D expertise and state-of-the-art analytical platforms.
Interchangeable Biosimilars: The Path to Pharmacy-Level Substitution
Within the biosimilar landscape, a distinct and higher-tier designation exists: the “interchangeable biological product.” An interchangeable biosimilar is a biosimilar that meets additional, more stringent requirements, allowing it to be substituted for the reference product at the pharmacy level without the intervention or approval of the prescribing healthcare provider. This mirrors the routine substitution of generic drugs for brand-name drugs and is a critical factor for widespread market penetration.
To achieve interchangeability status, manufacturers must provide additional data beyond what is required for biosimilarity. This typically involves “switching studies,” where patients are observed as they alternate between the reference product and the proposed interchangeable biosimilar. The results of these studies must conclusively demonstrate that there is no decrease in effectiveness and no increase in safety risk associated with such switching . Interchangeability is the ultimate market access differentiator for biosimilars, as it unlocks the potential for widespread, automatic substitution, mirroring the market dynamics of small-molecule generics. The ability for pharmacists to substitute an interchangeable biosimilar without requiring physician approval dramatically lowers the barrier to adoption, similar to how generic small molecules achieve high market penetration. This directly and significantly impacts sales volume and market share for the biosimilar product. While the FDA’s requirement for rigorous switching studies presents an additional hurdle and investment for developers, it is a necessary step to build the profound clinical confidence required for this level of automatic substitution, making it a highly valuable regulatory achievement.
Navigating Immunogenicity and Indication Extrapolation
Two significant scientific and regulatory considerations in biosimilar development are immunogenicity and indication extrapolation.
Immunogenicity refers to the propensity of a biological product to induce an immune response in patients, typically manifesting as the development of anti-drug antibodies (ADAs) . These ADAs can potentially influence the efficacy and/or safety profile of the therapeutic agent. Assessing immunogenicity is a complex challenge; it is difficult to predict accurately in preclinical models, as animal models often lack predictive value for human immunogenicity. Consequently, robust immunogenicity assessment primarily relies on clinical trials, often requiring long-term follow-up (e.g., 1-year follow-up for chronically administered agents) to detect neutralizing ADAs that may take time to develop . The goal of immunogenicity testing is to demonstrate that the risk of ADA development with a biosimilar is no greater than with the reference product.
Indication Extrapolation is a crucial regulatory concept that allows for the approval of a biosimilar product in multiple indications for which the reference product is already approved, without requiring extensive clinical trials for each individual indication . This approach is based on the principle that if a biosimilar demonstrates biosimilarity to a reference product in one sensitive indication, it can be extrapolated to other indications, provided there is sufficient scientific justification . This justification relies on a “totality of the evidence,” including a shared mechanism of action (MOA) across the indications, similar pharmacokinetic (PK) and pharmacodynamic (PD) profiles, comparable immunogenicity, and similar toxicity profiles . While widely accepted by major regulatory bodies like the FDA and EMA as a means to avoid unnecessary repetition of clinical trials , indication extrapolation remains a point of controversy among some learned societies who advocate for clinical data to be required for all indications . Successfully navigating immunogenicity and leveraging indication extrapolation are critical for biosimilar development, balancing scientific rigor with accelerated market access and cost efficiency. Immunogenicity represents a complex biological risk that can undermine a biosimilar’s safety and efficacy. Therefore, robust assessment and mitigation strategies are absolutely essential for ensuring patient safety and securing regulatory approval. Concurrently, indication extrapolation is a powerful tool for efficiency, allowing developers to avoid redundant and prohibitively costly clinical trials for every single indication. The inherent tension lies in providing sufficient scientific justification to satisfy regulators and build clinical confidence, without incurring the full development costs associated with a de novo biologic development program.
Regulatory Challenges for Complex Generics
The “complex generics” category, while offering significant market opportunities due to its less crowded nature, presents a unique and formidable set of regulatory and scientific hurdles that distinctly differentiate them from simpler generic drugs.
Drug-Device Combination Products: Unique Considerations
Complex generics frequently include drug-device combination products, where the drug constituent part is pre-loaded in, or specifically cross-labeled for use with, a product-specific device constituent part. Common examples include inhalers, autoinjectors (such as epinephrine injection autoinjectors), and certain pre-filled syringes . For these products, the design and performance of the device directly influence the drug’s delivery to the site of action or its absorption, introducing entirely new layers of complexity in demonstrating therapeutic equivalence .
Regulatory expectations for these combination products are intricate and extend far beyond standard bioequivalence requirements. They necessitate specific scientific studies that assess not only the drug’s performance but also the device’s functionality, its interaction with the drug, and its usability by patients. The development of generic drug-device combination products demands an interdisciplinary approach, integrating pharmaceutical science with engineering and device regulatory expertise, which can be a significant bottleneck. Unlike a simple pill, the efficacy of a drug-device combination product is intrinsically tied to both the drug’s inherent properties and the device’s precise performance. This means that generic developers must not only replicate the drug’s bioequivalence but also meticulously ensure that the device delivers the drug identically and safely. This requires specialized expertise in device design, manufacturing, and regulatory compliance, making it a more capital-intensive and specialized endeavor compared to traditional generic drug development.
Advanced Formulations: Liposomes, Nanoparticles, and Long-Acting Injectables
Modern pharmaceutical science increasingly employs advanced drug delivery systems to overcome challenges associated with new chemical entities, such as poor aqueous solubility, short half-life, or extensive degradation. These innovative formulations, including liposomes, polymer- or lipid-based nanoparticles, microspheres, and long-acting injectables (LAIs), are designed to improve drug delivery, reduce dosing frequency, and minimize adverse effects, thereby offering significant patient benefits.
However, developing generic versions of these complex formulations is exceptionally challenging. They often require extensive studies beyond traditional bioequivalence methods due to their intricate design, the use of specialized excipients, and highly complex manufacturing processes . For instance, generic transdermal patches, which deliver medication through the skin, require demonstrating not only bioequivalence but also statistical non-inferiority in terms of adhesion to the skin and the potential for skin irritation and/or sensitization compared to the reference product . Similarly, long-acting injectables (LAIs) present unique challenges related to high inter-subject pharmacokinetic variability, difficulties in patient recruitment for long-term studies, and the potential for drug carryover between study periods in crossover designs . The scientific and technical hurdles in replicating advanced formulations create a natural barrier to entry for generic developers, limiting competition and preserving higher profit margins for successful entrants. The inherent complexity of these formulations—involving multi-component systems, unique excipients, and intricate manufacturing processes—makes their characterization and replication incredibly difficult . This directly translates into higher research and development costs and significantly longer development timelines. Consequently, fewer generic companies possess the capabilities to successfully develop and bring these products to market, which inherently reduces competition and allows those who succeed to capture a larger share of the market value.
The Quest for Regulatory Clarity and Tailored Guidance
A significant impediment to the development and timely market entry of complex generics is the prevailing “lack of clarity around the regulatory pathway”. Unlike simpler generic products, there are often few specific FDA guidance documents tailored to these complex products. This leads to unclear expectations from regulatory agencies and continuously evolving requirements, which can result in significantly longer approval times than for standard generics .
The ambiguity in regulatory filing persists due to several factors: the use of complex formulations and excipients, the complicated characterization required for the drug products and their components, ill-defined in vitro drug release assay methods, and a scarcity of reliable models to establish in vivo pharmacokinetics. The absence of clear, product-specific regulatory guidance for complex generics acts as a significant bottleneck, increasing development risk and delaying patient access to potentially more affordable and convenient treatments. When regulatory expectations are vague, generic developers face considerable uncertainty, leading to increased R&D costs—for example, through iterative trial-and-error testing—and prolonged development timelines. This directly impacts a company’s speed-to-market and overall profitability. Furthermore, the lack of standardized testing methods makes it harder to prove therapeutic equivalence efficiently. This regulatory “vacuum” ultimately harms patients by delaying the availability of cost-effective alternatives for managing complex chronic conditions.
Global Perspectives: Comparing FDA and EMA Approval Processes
While the U.S. FDA and the European Medicines Agency (EMA) share the fundamental goal of ensuring the safety and efficacy of medicines, their approaches to generic and biosimilar approval exhibit both notable similarities and significant divergences. Understanding these differences is crucial for pharmaceutical companies pursuing global market strategies.
Similarities and Divergences in Regulatory Frameworks
Both the FDA and EMA demand rigorous scientific assessment to ensure the quality, safety, and efficacy of generic and biosimilar products. For biosimilars, both agencies require comprehensive head-to-head comparisons across pre-clinical, clinical, pharmacokinetic (PK)/pharmacodynamic (PD) profiles, and physicochemical characterization . Both also offer scientific consultations to sponsors throughout the drug development process to provide guidance and address specific questions.
However, several key divergences exist. The EMA often bases its biosimilar guidelines on the classification of the biological molecule, with specific policies available for different product classes, whereas the FDA tends to adopt a more case-by-case approach . Their geographical jurisdictions also differ, with the EMA governing the European Union, Norway, Iceland, and Liechtenstein, while the FDA regulates products for the U.S. market. Furthermore, the FDA possesses direct authority to approve drug products in the U.S., whereas the EMA evaluates submissions and provides non-binding recommendations to the European Commission, which then makes the final legally binding decision. Exclusivity periods for biologics also vary, with 12 years in the USA and 10 years in the EU . These divergent regulatory requirements between major markets like the U.S. and EU create market fragmentation, significantly increasing development costs and complexity for generic companies aiming for global launches. If a generic or biosimilar developer intends to launch a product in both the U.S. and EU, they must navigate two potentially different sets of requirements. This can lead to redundant studies, necessitate different trial designs (e.g., involving distinct patient populations to meet specific regional criteria ), and substantially increase the complexity of regulatory submissions. This directly translates to higher research and development costs and longer overall development timelines, making global market entry a more challenging and less efficient endeavor.
Collaborative Initiatives: Towards Harmonized Development
Recognizing the challenges posed by differing regulatory requirements and the desire to streamline global drug development, both the FDA and EMA have engaged in collaborative initiatives. A notable example is the Parallel Scientific Advice (PSA) pilot program for complex generic and hybrid products, which was launched in September 2021 .
This program provides a mechanism for assessors from both agencies to concurrently exchange their views on scientific issues with applicants during the development phase of complex generic drug and hybrid products. The overarching goal of such collaboration is to drive convergence in regulatory expectations, help applicants avoid redundant work and unnecessary testing, and ultimately shorten the time to drug development and approval. International regulatory collaboration, such as the FDA/EMA PSA program, is a crucial mechanism for streamlining complex generic and biosimilar development, ultimately accelerating patient access globally. By providing concurrent scientific advice, the PSA program helps generic companies design studies and development programs that are acceptable to both major regulatory bodies from the outset. This significantly reduces the likelihood of needing to conduct separate, potentially conflicting studies, thereby cutting down on time, cost, and regulatory risk. Even partial harmonization directly translates to faster market entry and broader availability of affordable medicines for patients worldwide.
| Feature | U.S. FDA Approval (ANDA/505(b)(2)/351(k)) | European Medicines Agency (EMA) Approval (Centralized Procedure) |
| Primary Pathways | ANDA (Small Molecule Generics), 505(b)(2) (Hybrid), 351(k) (Biosimilars) | Centralized Procedure for Generics/Hybrids/Biosimilars (via Marketing Authorization Application) |
| Governing Legislation | Hatch-Waxman Act (1984), BPCIA (2010) | Directive 2001/83/EC, Regulation (EC) No 726/2004 |
| Drug Types Covered | Small Molecule Generics, Complex Generics, Biosimilars | Small Molecule Generics, Hybrid Medicines (includes many complex products), Biosimilars |
| Key Equivalence Standard | Bioequivalence (ANDA), Highly Similar (351(k)), Bridging (505(b)(2)) | Bioequivalence (Generics), Highly Similar (Biosimilars/Hybrids) |
| Clinical Trial Requirement | Abbreviated (no repeat full clinicals for ANDA/351(k)), targeted studies for 505(b)(2)/351(k) | Abbreviated (no repeat full clinicals), targeted comparability studies for biosimilars/hybrids |
| Typical Development Cost | $1-4M (simple generic), $100M+ (biosimilar) | Generally comparable to US, but specific fees may vary |
| Exclusivity Period for Biologics | 12 years | 10 years |
| Interchangeability Designation | Specific “Interchangeable Biosimilar” designation requiring switching studies | EMA/HMA state biosimilars are scientifically interchangeable; national decisions on pharmacy substitution |
| Regulatory Approach | More case-by-case for biosimilars/complex generics | Often classification-based guidelines for biological molecules |
| Decision Authority | FDA has direct approval authority | EMA provides non-binding recommendation to European Commission for final decision |
| Collaborative Initiatives | Parallel Scientific Advice (PSA) with EMA | Parallel Scientific Advice (PSA) with FDA |
Table 2: Key Regulatory Pathways Comparison (FDA vs. EMA)
Intellectual Property and Market Dynamics: The Strategic Battlefield
The pharmaceutical industry is a complex ecosystem where scientific innovation, regulatory rigor, and fierce market competition converge. At the heart of this dynamic lies intellectual property, particularly patents, which serve as both protectors of innovation and shapers of competitive dynamics. For generic drug developers, understanding this strategic battlefield is paramount to identifying opportunities and navigating potential pitfalls.
Pharmaceutical Patents: Protecting Innovation and Shaping Competition
Patents are the lifeblood of pharmaceutical innovation, providing a crucial incentive for the enormous investment required for novel drug discovery and development. They grant inventors exclusive rights to their inventions for a set period, typically 20 years from the date on which the patent application was filed in the United States . This period of exclusivity allows innovator companies to recoup their substantial R&D costs and generate profits, which can then be reinvested into further research.
Types of Patents: Compound, Formulation, Method of Use, Polymorph
Pharmaceutical companies employ a multifaceted approach to intellectual property protection, strategically layering various types of patents to safeguard their innovations and extend their market exclusivity.
- Compound Patents: These are the foundational patents, protecting the active chemical entity itself. They are typically the earliest patents filed and provide the broadest protection for the drug.
- Formulation Patents: Innovator companies frequently patent new formulations of an existing drug. A prime example is AstraZeneca’s Seroquel XR, an extended-release, once-a-day quetiapine formulation . Such formulations can offer significant patient benefits, like reduced dosing frequency, and if successfully launched and established before the basic compound patent expires, they can provide valuable additional exclusivity.
- Method of Use Patents: These patents protect specific approved indications or ways of using the drug. For instance, a drug might be patented for treating a particular disease or condition, even if the compound itself is known. These patents are particularly relevant for “skinny-labeling” strategies by generic manufacturers.
- Polymorph Patents: A drug compound can exist in various crystalline structures, known as polymorphs, which share the same chemical composition but differ in their molecular arrangements. These variations can lead to differing physical properties such such as solubility and dissolution rates . Patenting new polymorphs can effectively extend patent protection for a drug.
Innovator companies strategically layer multiple types of patents to create “patent thickets,” effectively extending their market exclusivity and delaying generic entry beyond the initial compound patent. A 20-year compound patent is often not the end of a drug’s exclusive market life. By subsequently patenting new formulations, methods of use, or polymorphs, brand companies can construct a dense and overlapping web of intellectual property rights . This practice, often referred to as “evergreening” , means that even if the primary compound patent expires, generic manufacturers may still find their market entry blocked by these ancillary patents. This forces generic companies into costly and time-consuming litigation or significantly delays their ability to bring affordable alternatives to market. This is a deliberate and sophisticated strategy employed by innovator companies to maximize their revenue streams.
Patent Term and Exclusivity Periods: A Critical Timeline
Beyond the standard patent terms, the FDA grants various types of exclusivity upon drug approval, which also serve to protect brand-name drugs from generic competition . It is important to distinguish that these exclusivities are statutory and are not added to the patent life; rather, they run concurrently or independently of existing patents . Key types of exclusivity include:
- Orphan Drug Exclusivity (ODE): Provides 7 years of market protection for drugs developed to treat rare diseases or conditions affecting fewer than 200,000 people in the U.S. .
- New Chemical Entity (NCE) Exclusivity: Grants 5 years of exclusivity for a drug that contains no active moiety (the molecule or ion responsible for the drug’s physiological or pharmacological action) that has been previously approved by the FDA .
- “Other” Exclusivity: Provides 3 years of exclusivity for changes to an already approved drug, such as a new indication, a new dosage form, or a new route of administration, provided that new clinical studies were conducted to support the approval of that change .
- Pediatric Exclusivity (PED): Adds 6 months to any existing patents and exclusivities on a drug if the sponsor conducts pediatric studies in response to a written request from the FDA .
- 180-Day Exclusivity: A crucial incentive under the Hatch-Waxman Act, granted to the “first” generic applicant who submits a substantially complete Abbreviated New Drug Application (ANDA) containing a Paragraph IV certification, thereby challenging the validity or infringement of a listed patent .
For generic developers, accurately tracking patent expiration dates and exclusivity periods is the ultimate competitive intelligence, determining the optimal window for market entry and maximizing the return on investment. The interplay of different patent types and various exclusivity periods creates an exceptionally complex “patent landscape.” A generic company’s success critically hinges on precisely identifying when all these intellectual property protections expire or can be legally challenged. This precise timing dictates when an ANDA can be filed and, crucially, when market entry becomes possible. This directly impacts the ability to secure the highly coveted “first-to-file” advantage and the potential for significant initial profits before other generic competitors enter the market. This is precisely where specialized tools, such as DrugPatentWatch, become indispensable for providing the necessary competitive intelligence.
Evergreening Strategies: Brand-Name Tactics to Delay Generic Entry
“Evergreening” refers to a range of legal, business, and technological strategies employed by brand-name pharmaceutical companies to extend the patent lifetime of their drugs, thereby retaining revenues and delaying the entry of generic competition . These tactics are often framed as “lifecycle management” but are frequently criticized for their anti-competitive effects.
Product Hopping and Minor Modifications
A common evergreening tactic is “product hopping” or “product switching,” where a brand-name company attempts to extend its patent, and consequently its revenue stream, by patenting minor variations of the original product. This can manifest in several ways:
- Changes to Dosage Form: Altering the drug from, for example, a capsule to a tablet, or vice versa.
- Chemical Changes: Introducing slight chemical modifications, such as adjusting chemical groups (a “me-too” drug) or a different enantiomeric mixture (a “chiral switch”).
- Formulation Changes: Switching from short-acting to long-acting formulations to reduce dosing frequency .
- New Inactive Ingredients or Combinations: Introducing new excipients or combining the drug with other active ingredients .
While some of these modifications can represent genuine improvements that offer meaningful benefits for patients (e.g., an easier route of administration), they are considered anti-competitive if their primary purpose is to delay generic market entry. Notable examples include the multiple sclerosis drug glatiramer acetate (Copaxone) and the Alzheimer’s drug memantine (Namenda), where product hopping tactics led to billions in additional costs for consumers before patents were challenged or invalidated. Product hopping blurs the line between genuine pharmaceutical innovation and anti-competitive behavior, forcing generic companies to invest heavily in legal challenges rather than solely in research and development. When a brand company introduces a slightly modified version of a drug just before its primary patent expires, it can effectively compel patients and prescribers to switch to the new version. This maneuver can render the original, now off-patent, version obsolete in the market, thereby delaying generic uptake. This strategic shift transforms the competitive battleground from one of scientific development to one dominated by legal disputes, requiring generic firms to allocate significant resources to challenging these new patents. Such legal battles can be a costly and time-consuming distraction from their core mission of bringing affordable medicines to market.
Patent Thickets and Legal Challenges
Brand-name companies frequently employ the strategy of creating “patent thickets”—a dense and overlapping web of patent rights on active substances, formulations, or even non-essential features of a drug—to deter generic entry . This tactic is often combined with “linkage evergreening,” a process where pharmaceutical regulators are required to “link” their evaluation of an impending generic product with an assessment of whether it might infringe an existing patent . This complex interplay of patents and regulatory processes significantly complicates the landscape for generic manufacturers.
These aggressive intellectual property tactics frequently lead to patent litigation in federal court. If the brand-name drug sponsor timely files a lawsuit following a generic company’s Paragraph IV certification (a claim that the generic drug does not infringe or that the patent is invalid), the FDA is generally prevented from approving the ANDA for 30 months while the litigation proceeds. This is known as the “30-month stay,” and it effectively delays generic market entry . Another controversial tactic employed by brand companies is “reverse payment settlements” or “pay-for-delay” agreements, where the brand company pays the generic firm to delay its market entry. The pharmaceutical patent landscape is often a battleground of legal strategies, where generic companies must be prepared for aggressive patent enforcement and litigation as an inherent part of their business model. The high stakes involved in pharmaceutical revenue streams mean that brand companies will vigorously defend their intellectual property. This transforms generic market entry into a complex legal chess match, frequently involving Paragraph IV challenges and subsequent patent infringement lawsuits. Generic firms must therefore possess strong legal departments or engage expert external counsel, coupled with a deep understanding of patent law, to successfully navigate these challenges and secure their market opportunities. This is not merely a regulatory process but a full-blown legal contest.
The Patent Cliff: A Recurring Opportunity for Generic Manufacturers
The term “patent cliff” vividly describes a critical phenomenon in the pharmaceutical industry: situations where multiple high-revenue, blockbuster brand-name drugs lose patent protection within a compressed timeframe . This event creates a massive and predictable opportunity for generic manufacturers, as it fundamentally transforms market dynamics by dissolving exclusivity protections and enabling the rapid entry of generic competition .
When patents expire, generic alternatives typically enter the market at significantly reduced price points, often initially around 30% of the original product’s cost . As additional generic manufacturers join the competition, prices frequently decline further, sometimes plummeting to as little as 10-20% of the original branded price . The patent expiration of the cholesterol-lowering drug Lipitor in 2011 serves as a prime historical example of this market transformation, leading to the widespread availability of affordable generic versions . The patent cliff is the cyclical engine of the generic pharmaceutical industry, dictating major strategic shifts and creating predictable windows of high-value opportunity. Generic companies can anticipate these patent expirations years in advance. This predictability allows for long-term strategic planning, encompassing R&D investment, manufacturing scale-up, and meticulous legal preparation for Paragraph IV challenges. The “cliff” represents a recurring, high-impact event that profoundly reshapes market share and revenue streams across the industry, making it the most significant external driver for generic business strategy.
Competitive Dynamics in the Generic Market
The generic pharmaceutical market is characterized by intense and often aggressive competition, which serves as the primary mechanism for driving down drug prices and increasing patient access to essential medicines.
The “First-to-File” Advantage and 180-Day Exclusivity
The Hatch-Waxman Act provides a powerful incentive for generic companies to be the “first-to-file” an Abbreviated New Drug Application (ANDA) that includes a Paragraph IV certification, thereby challenging the validity or enforceability of a brand-name drug’s patent . If this generic applicant successfully prevails in challenging the patent, they are granted a coveted period of 180 days of market exclusivity .
This 180-day exclusivity can be incredibly lucrative for the first generic entrant. During this period, the company has the exclusive right to market its generic drug, often pricing it strategically slightly below the branded version. This allows them to capture a significant portion of the market share and maintain higher profit margins before other generic competitors are able to enter the market and inevitably erode prices. This powerful financial incentive has transformed Paragraph IV certifications into a routine and highly competitive part of generic business strategy, with a steady uptick in such cases over the years. The “first-to-file” 180-day exclusivity is the generic industry’s equivalent of a gold rush, incentivizing aggressive patent challenges and rewarding early movers with a significant, albeit temporary, monopoly. The financial benefits of being the sole generic competitor for 180 days are substantial, enabling the first entrant to establish a strong market presence and capture significant revenue before the inevitable price collapse that follows the entry of additional competitors. This creates a fierce race to be the first, driving considerable investment in patent intelligence and legal expertise. It is a high-risk, high-reward strategy that profoundly defines much of the competitive landscape within the generic pharmaceutical sector.
Price Erosion with Increased Competition: A Race to the Bottom?
Once a generic drug successfully enters the market, drug prices typically fall substantially. The entry of even a single generic competitor can lead to a price reduction of approximately 39% compared to the brand-name drug. This price reduction accelerates dramatically with increased competition: with two generic competitors, prices can fall by 54%, and with six or more generic entrants, prices can plummet by a staggering 95% . This aggressive price erosion is a direct consequence of the competitive dynamics fostered by the Hatch-Waxman Act.
However, this intense competition and rapid price erosion also create significant challenges for generic manufacturers. Prices can be driven so low that they become unprofitable to produce, particularly for older, small-market medicines or complex sterile injectables . When profitability diminishes, manufacturers may decide to cease production, leading to market exits and a reduction in the number of suppliers. This, in turn, can make the supply chain less diversified and more prone to drug shortages . While robust generic competition is vital for patient affordability, the resulting aggressive price erosion can undermine the long-term sustainability of the generic manufacturing sector, leading to market exits and drug shortages. The very mechanism designed to lower costs—intense competition—can, if left unchecked, lead to a “race to the bottom” where profit margins become unsustainable . When drugs become unprofitable to manufacture, companies are compelled to exit the market, reducing the number of suppliers and making the supply chain vulnerable to critical shortages . This highlights a critical policy dilemma: how to maintain affordability without jeopardizing the stability and resilience of the generic drug supply, especially for essential medications used in chronic disease management.
| Number of Generic Competitors | Average Price Reduction Compared to Brand (%) | Context / Source |
| 1 | 39% | Initial entry |
| 2 | 54% | After second generic entry |
| 3-5 | 15-40% (additional savings) | After first generic entry |
| 6+ | 95% | After multiple generic entries |
Table 3: Impact of Generic Competition on Drug Prices
Overcoming Challenges in Generic Drug Development and Commercialization
The journey from a patent expiration to a successfully commercialized generic drug is fraught with challenges. Beyond the regulatory and intellectual property hurdles, generic manufacturers must contend with complexities in manufacturing, supply chain vulnerabilities, intricate reimbursement policies, and the crucial realm of patient and physician perceptions. Overcoming these obstacles requires strategic foresight, operational excellence, and a deep understanding of the broader healthcare ecosystem.
Manufacturing Excellence and Quality Assurance
Maintaining impeccable manufacturing quality is not merely a regulatory compliance exercise for generic drugs; it is a fundamental pillar of patient trust, product reputation, and ultimately, market success. Generic medications must meet the same rigorous quality standards as their brand-name counterparts .
Adherence to Current Good Manufacturing Practices (cGMP)
The FDA ensures the quality of drug products by meticulously monitoring drug manufacturers’ compliance with its Current Good Manufacturing Practice (cGMP) regulations. These regulations establish the minimum requirements for the methods, facilities, and controls used in the manufacturing, processing, and packing of a drug product. Their purpose is to ensure that every product is safe for use, contains the ingredients and strength it claims to have, and is consistently produced with the required quality . FDA assessors and investigators conduct thorough reviews of manufacturing procedures and perform on-site inspections of generic drug manufacturing facilities to verify capabilities and the accuracy of submitted information . This stringent oversight is designed to guarantee product consistency and reliability.
In a commoditized market, cGMP compliance and a robust quality reputation are not just regulatory necessities but critical competitive differentiators, directly influencing market share and mitigating the risks of costly recalls. When prices are driven down, as is common in the generic sector, there can be a perception that quality might be compromised, especially with concerns frequently raised about overseas manufacturing . Companies that consistently demonstrate superior cGMP compliance and unwavering quality assurance build profound trust with healthcare providers and payers. This established reputation can translate into preferred formulary placement and higher prescribing rates, offering a significant competitive edge that extends beyond mere price. Conversely, any lapse in quality or a significant manufacturing issue can lead to severe reputational damage, widespread product recalls, and substantial financial losses . As Cynthia Fanning, Vice President at GE Appliances, aptly noted, “To be competitive, we have to look for every opportunity to improve efficiencies and productivity while increasing quality. Lean manufacturing principles have improved every aspect of our processes” .
Quality Control Systems and Post-Market Surveillance
Robust quality control (QC) measures are essential throughout the entire manufacturing process, encompassing in-process testing and final product testing, to ensure the pharmacological potency, purity, and stability of generic drugs . Analytical methods must be meticulously validated, and batch-to-batch consistency rigorously monitored to ensure that every lot of the product meets the defined quality specifications .
Beyond pre-market approval, post-market surveillance plays an increasingly vital role in continuously monitoring the safety, efficacy, and usage patterns of generic drugs in real-world settings . This involves systematic analysis of various data sources, including adverse event reporting systems (such as the FDA Adverse Event Reporting System, FAERS) and comprehensive healthcare claim databases, to identify any potential issues or biases related to generic drug use . Post-market surveillance is an extension of quality control, providing crucial real-world data that can either reinforce or, in some cases, challenge the scientific assumptions of bioequivalence, necessitating continuous monitoring and responsiveness from generic manufacturers. While pre-market approval focuses on demonstrating bioequivalence under controlled conditions, real-world usage can sometimes reveal subtle differences or unexpected adverse events . Post-market surveillance acts as an early warning system, allowing regulators and manufacturers to detect and investigate such signals. For generic companies, proactively monitoring this data and addressing any reported issues, even if anecdotal or perception-based, is vital for maintaining product integrity, ensuring ongoing regulatory compliance, and preserving public trust, especially given existing patient and physician perceptions about generics.
Innovations in Manufacturing: Continuous Processing and Automation
Traditional batch manufacturing processes, which have long been the standard in pharmaceuticals, are often characterized by inherent inefficiencies, lengthy cycle times, and susceptibility to variability. Innovations like continuous manufacturing offer a transformative solution to these challenges. This advanced approach integrates multiple discrete steps of the manufacturing process into a single, uninterrupted system, moving raw materials through the process continuously to produce a finished product .
The benefits of continuous manufacturing are manifold. It can lead to significantly improved quality control through real-time monitoring, utilizing technologies such as Process Analytical Technology (PAT). This allows for immediate detection and correction of deviations, enhancing product consistency and reducing batch failures. Furthermore, continuous manufacturing can result in a reduced manufacturing footprint, faster production times, and greater flexibility to respond to demand fluctuations . The FDA has explicitly recognized continuous manufacturing as a key technology that can help prevent drug shortages caused by product quality and manufacturing problems. Investing in advanced manufacturing technologies like continuous processing offers generic manufacturers a pathway to overcome persistent cost pressures and quality concerns, thereby transforming operational efficiency into a strategic competitive advantage. The “razor-thin profit margins” that characterize much of the generic market make traditional manufacturing economically challenging. Continuous manufacturing, by significantly reducing direct labor costs, minimizing material waste, and drastically shortening cycle times (e.g., from 30 days to 5 days for some drugs) , can substantially lower production costs and improve overall profitability. This allows manufacturers to remain competitive while simultaneously enhancing product quality and reducing the risk of critical drug shortages, creating a powerful differentiator in the market.
Supply Chain Resilience and Addressing Drug Shortages
The generic drug supply chain, while indispensable for ensuring affordable access to medicines, is inherently complex, globally dispersed, and vulnerable to disruptions. This inherent fragility frequently leads to drug shortages, which can have profound and detrimental impacts on patient care, particularly for individuals managing chronic diseases.
Vulnerabilities in the Global Generic Drug Supply Chain
Generic drugs account for nearly 90% of all prescriptions dispensed in the U.S. , yet they are disproportionately affected by shortages. Data from 2017 to 2021 shows that the FDA received 731 “supply chain issue reports” from manufacturers, with 113 of these leading to “meaningful” shortages. A substantial majority of these shortages involved generic drugs, and many lasted for more than a year.
The root causes of these vulnerabilities are deeply embedded in the economic structure of the generic drug market. Intense price competition drives down profit margins to unsustainable levels, which in turn disincentivizes manufacturers from investing in crucial resilience measures such as spare production capacity or maintaining adequate buffer inventories. This economic pressure leads to a concentrated supply base, where a small number of firms produce a significant portion of essential generic drugs. Such concentration makes the supply chain brittle and highly susceptible to disruptions; any issue experienced by a single supplier can have a large and immediate impact on total market supply . Sterile injectable drugs, due to their intricate manufacturing processes and often limited number of suppliers, are particularly vulnerable and tend to experience longer-lasting shortages, with a median duration of 4.6 years . The economic structure of the generic drug market, driven by fierce price competition, inadvertently creates systemic vulnerabilities in the supply chain, transforming cost-saving measures into a public health risk. The relentless downward pressure on generic prices means that manufacturers operate on minimal margins. This directly disincentivizes investments in redundancy, such as establishing multiple manufacturing sites or maintaining buffer stocks of raw materials and finished products, as well as disincentivizes modernization of facilities . When production is concentrated among a few low-profit manufacturers, any disruption—be it a quality issue, a natural disaster, or a geopolitical event—can quickly cascade into widespread shortages, directly impacting the availability of essential medicines and leading to adverse patient outcomes . This highlights a critical market failure where short-term cost savings lead to long-term supply instability.
Strategies for Diversification and Domestic Manufacturing
To build a more robust and resilient pharmaceutical supply chain, a multi-pronged strategic approach is essential. Key strategies include diversifying the supply base, which involves both establishing redundancy in manufacturing capacity and achieving a balanced mix of domestic and diversified foreign sourcing. Furthermore, there is a critical need for sustained investment in reliable, efficient, and sustainable manufacturing practices across the industry.
There is a growing and concerted push to bring more generic drug manufacturing back to the United States. This strategic shift aims to reduce the nation’s dependence on overseas suppliers, mitigate potential quality concerns that have historically arisen from some foreign manufacturing sites, and ensure a more predictable supply and pricing for essential medicines . Addressing generic drug shortages requires a concerted policy shift towards incentivizing supply chain resilience and domestic manufacturing, moving beyond a sole focus on lowest-cost procurement. The current market incentives are often not aligned to ensure adequate investment in supply chain resilience. Therefore, proactive policy interventions are crucial. Promoting domestic manufacturing, building national inventories of priority generics, and establishing robust public-private partnerships are direct and necessary responses to the identified vulnerabilities. These measures aim to create a more secure and stable supply of essential medicines, even if it entails a slight increase in initial production costs, prioritizing long-term stability and patient safety over immediate, short-term price reductions.
Case Studies of Shortages and Their Impact on Chronic Disease Management
Generic drug shortages are not abstract economic issues; they have tangible, severe consequences for patient health, underscoring the moral and business imperative for supply chain stability. A stark illustration of this is the case of valsartan, a widely used generic drug for high blood pressure. In 2018, carcinogenic contamination led to a major FDA recall of valsartan, resulting in a serious and prolonged shortage that lasted for years. Even though other blood pressure medications were available, they were not perfect substitutes for all patients, and the health effects of this shortage were substantial.
Beyond individual cases, broader analyses consistently demonstrate the severe impact of drug shortages. Studies have linked drug shortages to elevated mortality rates, an increased incidence of adverse reactions, and higher out-of-pocket costs for patients . For essential medicines, which are critical for managing chronic conditions, shortages tend to last significantly longer, with a median duration of 4.0 years, compared to 2.3 years for non-essential medicines . The valsartan case is a potent reminder that failures within the supply chain translate directly into patient harm, potentially including increased morbidity and mortality . For individuals living with chronic diseases who require continuous medication, a shortage can force them to switch therapies, which may lead to adverse events, sub-optimal disease control, or increased anxiety. This emphasizes that supply chain resilience is a core component of patient care and a critical responsibility for generic manufacturers, extending beyond mere commercial considerations.
Reimbursement Policies and the Role of Pharmacy Benefit Managers (PBMs)
Reimbursement policies and the practices of Pharmacy Benefit Managers (PBMs) exert a profound influence on the accessibility and affordability of generic drugs. While PBMs are intended to manage prescription drug benefits and control costs, their complex practices often create dynamics that significantly impact both generic manufacturers and individual patients.
Formularies and Cost-Sharing: Impact on Patient Access
Pharmacy Benefit Managers (PBMs) play a central role in the U.S. healthcare system, managing prescription drug benefits on behalf of health insurers. Their functions include negotiating prices with drug manufacturers and pharmacies, and, crucially, designing formularies—the lists of covered drugs—which dictate patient access to different medications and their associated out-of-pocket costs.
While generic drugs are fundamentally designed to be cost-effective, patients can still face significant and variable out-of-pocket expenses. For instance, in 2022, while most Medicare Part D enrollees filled at least one generic prescription for $2 or less, a substantial 54% paid more than $2 for at least one generic fill, and over 12% paid more than $20 for a generic prescription. This variability in patient out-of-pocket costs for generics, heavily influenced by PBM formularies and benefit designs, can still create significant affordability barriers, thereby undermining the full potential of generics to improve medication adherence and advance health equity. Despite generics being inherently cheaper, the final price a patient pays at the pharmacy counter can remain substantial due to complex PBM pricing models and the tiered structure of formularies . This means that even when a generic alternative is available, the cost can still deter adherence, particularly for low-income or uninsured patients, who are already more likely to skip or postpone care due to cost . This highlights that the “affordability” promise of generics is not always fully realized at the patient level due to the complexities introduced by intermediaries in the supply chain.
PBM Practices: Rebates, Spread Pricing, and Their Influence on Generic Uptake
PBMs generate revenue from various sources, including a share of the drug rebates they negotiate with pharmaceutical companies and a practice known as “spread pricing.” Spread pricing involves charging insurers a higher amount for a given drug than what the PBM actually reimburses the pharmacy, pocketing the difference.
Concerns have been raised that certain PBM practices, particularly concerning generics, can inflate costs rather than reduce them. Studies suggest that some PBMs overpay pharmacies for generics—in some instances, as high as 10 times the acquisition costs for certain drugs like Imatinib and Aripiprazole in Medicare Part D—and then recover significant portions of this through opaque “clawbacks”. This inflation of point-of-sale reimbursement can lead to higher patient cost-sharing, as patient out-of-pocket costs are often based on this inflated reimbursement amount. The opaque financial practices of PBMs, particularly “spread pricing” and generic over-reimbursement, distort the true cost-saving potential of generics, creating inefficiencies and potentially higher costs for patients and payers. The PBM layer in the pharmaceutical supply chain introduces significant complexity and a pervasive lack of transparency . When PBMs profit from the “spread” or by over-reimbursing pharmacies for generic drugs and then subsequently clawing back funds, the intended savings generated by generic competition are not fully passed on to patients or health plans . This creates a fundamental misalignment of incentives that can undermine the very purpose of generic drugs: to provide affordable and accessible treatment options.
Policy Reforms and Their Potential Impact on Generic Affordability
In response to growing concerns about drug costs and PBM practices, policymakers at both federal and state levels are actively considering various reforms to regulate PBMs. These proposed reforms include requiring greater transparency around rebates, delinking rebates from drug list prices, standardizing contracting terms and practices, and banning spread pricing. The aim of these measures is to ensure that more of the savings generated by generic competition are passed on to patients and payers.
However, new legislation, such as the Inflation Reduction Act (IRA), could also have complex and potentially unintended consequences on the generic market. While the IRA aims to lower drug costs through mechanisms like Medicare drug price negotiation, it may inadvertently reduce incentives for generic entry. If negotiated brand drug prices (Maximum Fair Prices, MFPs) significantly narrow the price differential between brand-name and generic drugs, it could make generic competition less financially attractive for manufacturers. Policy reforms targeting PBM transparency and pricing practices are crucial for unlocking the full cost-saving potential of generic drugs. However, new legislation like the IRA could have unintended consequences on generic market incentives. To ensure that generics deliver their maximum value, legislative action is indeed needed to increase transparency and curb practices that inflate costs. However, policymakers must carefully consider the ripple effects of new laws. If the IRA’s drug price negotiation program significantly reduces brand drug prices, it could inadvertently make generic entry less profitable, potentially deterring generic manufacturers from investing in new generic products and thereby reducing future competition. This necessitates a delicate balance in policy design to avoid solving one problem by inadvertently creating another.
Patient and Physician Perceptions: Building Trust and Enhancing Adherence
Despite the robust scientific evidence of bioequivalence and the rigorous regulatory oversight, patient and physician perceptions of generic drugs can significantly influence their acceptance, and consequently, patient adherence to medication regimens and overall health outcomes.
Misconceptions About Generic Efficacy and Safety
A persistent challenge in the generic drug market is the presence of misconceptions among some patients and even healthcare providers regarding the efficacy and safety of generic medications. These perceptions often include beliefs that generics are “less potent,” “not as good as the real medicine,” or that they require higher doses and consequently result in more side effects . Such negative perceptions are particularly prevalent among certain demographic groups, including the elderly, minorities, and individuals with lower socioeconomic status and health literacy .
While the FDA officially maintains that all approved generic drugs are just as safe and effective overall as their brand-name counterparts , the agency is aware of reports of “undesired effects” when patients switch from a brand-name drug to a generic formulation, or from one generic to another. For drugs with a narrow therapeutic index—where small changes in dosage or bioavailability can lead to significant differences in therapeutic effect or increased toxicity—even minor variations in formulation or absorption profile could theoretically result in subtherapeutic levels or increased adverse events. The disconnect between the scientific equivalence of generics and persistent patient/physician misconceptions creates a significant barrier to optimal generic uptake and adherence, necessitating targeted educational interventions. The FDA’s rigorous approval process is meticulously designed to ensure bioequivalence. However, patient trust is not solely built on scientific data. Factors such as differences in the appearance of pills (color, shape, size) or changes in packaging can cause anxiety, insecurity, and confusion among certain patient populations , which can directly lead to reduced adherence. This highlights that for generic drugs to fully deliver their public health benefit, the industry and broader healthcare system must actively address these psychological and informational barriers, not just the scientific ones.
Communication Strategies to Promote Generic Acceptance
Effective communication from healthcare professionals plays a pivotal role in increasing generic drug use. This involves proactively educating patients about the scientific basis of bioequivalence, reassuring them about the safety and efficacy of generics, and clearly explaining the substantial cost-saving benefits they offer . Such open dialogue can help dispel myths and build confidence.
For generic manufacturers, transparent labeling and a consistent commitment to quality can significantly help in building a strong reputation and fostering trust among both prescribers and patients . Furthermore, leveraging modern digital marketing strategies, such as search engine optimized (SEO) content, targeted online advertisements, and educational webinars, can amplify reach and effectively influence prescribing patterns and patient choices . For generic companies, investing in robust communication and educational campaigns for both healthcare professionals and patients is a crucial, often overlooked, strategy to drive adoption and overcome ingrained biases. Simply having an approved generic product is not sufficient if patients and prescribers are hesitant to utilize it. Proactive education can effectively dispel common myths and build confidence in generic medications, which directly translates to higher prescription rates and improved patient adherence. This approach moves beyond a purely transactional sales model to one focused on building long-term trust and understanding within the broader healthcare ecosystem.
The Link Between Affordability, Adherence, and Health Outcomes
The affordability provided by generic drugs is directly and profoundly linked to improved medication adherence. When out-of-pocket costs for prescriptions are reduced, patients are demonstrably more likely to fill their prescriptions and consistently take their medications as prescribed . This financial accessibility removes a significant barrier to consistent treatment.
Studies consistently demonstrate that medication non-adherence leads to a cascade of negative consequences: worse clinical outcomes, a lower quality of life for patients, and significantly higher overall healthcare costs, estimated at an astounding $300 billion per year in the U.S. healthcare system. Therefore, actively promoting the use of generic drugs is a direct and impactful pathway to achieving better health outcomes for individuals and advancing overall health equity within communities. The ultimate value proposition of generic drugs for chronic diseases lies in their profound ability to translate cost savings into tangible improvements in patient health and system efficiency through enhanced medication adherence. This perspective highlights that the benefit of generics extends far beyond merely saving money; it is about empowering patients to afford and consistently take their medications. This, in turn, directly impacts the progression of their chronic diseases, significantly improves their quality of life, and substantially reduces the financial and operational burden on the healthcare system. This holistic view powerfully reinforces why generic drug development is not just a commercial endeavor, but a strategic imperative with widespread societal benefits.
Strategic Opportunities and the Future of Generic Drugs for Chronic Diseases
As the pharmaceutical landscape continues to evolve, the generic drug sector is poised for significant growth and transformation. Beyond merely replicating existing brand-name drugs, strategic opportunities are emerging in areas that demand innovation, technological integration, and a deeper understanding of market dynamics and patient needs. Identifying these high-potential areas and adopting forward-thinking strategies will be key to future success.
Identifying High-Potential Therapeutic Areas for Generic Development
Strategic generic drug development begins with a meticulous assessment of therapeutic areas that are characterized by a confluence of high unmet medical needs, a significant global disease burden, and the impending expiration of key patents.
Cardiovascular Diseases, Diabetes, Respiratory Conditions, and Beyond
Chronic diseases such as cardiovascular diseases (e.g., hypertension, hyperlipidemia), diabetes, and chronic respiratory diseases (e.g., asthma, COPD) account for a large proportion of global Noncommunicable Disease (NCD) deaths. These therapeutic areas consistently represent high-volume markets with a continuous demand for long-term medication, making them prime targets for generic development as patents expire. For example, atorvastatin calcium (for cholesterol), amlodipine besylate (for hypertension), and metformin HCl (for diabetes) are among the most prescribed generic drugs in the U.S. . The prevalence of these conditions ensures a large and consistent patient population, which translates directly into significant market volume once generic versions become available. Therapeutic areas with high chronic disease burden and impending patent expirations represent the most attractive targets for generic development, promising significant market volume and public health impact. The direct correlation between the widespread prevalence of these chronic diseases and the sustained demand for their management creates a clear and compelling market opportunity for generic manufacturers. These are not niche markets but foundational segments of healthcare where affordability can make a profound difference in patient access and outcomes.
Unmet Medical Needs and Market Gaps
Beyond sheer volume, strategic generic development also focuses on identifying and addressing specific unmet medical needs and existing market gaps within chronic disease management. This involves targeting therapeutic areas with high incidence, significant mortality, and profound quality-of-life deficits. Oncology (cancer), neurological disorders (including neurodegenerative diseases), metabolic and endocrine disorders (like diabetes), infectious diseases, and autoimmune diseases are consistently highlighted as fields with substantial attention in drug R&D pipelines due to their immense public health implications and potential for novel interventions .
Within these broad categories, opportunities exist for complex generics that address specific patient challenges, such as medication non-adherence. For instance, the development of long-acting injectable (LAI) formulations for conditions like schizophrenia can significantly improve adherence by reducing the frequency of dosing, thereby addressing a documented problem in the treatment of this condition. Identifying and addressing unmet medical needs within chronic disease management, particularly through complex generic formulations, allows companies to carve out strategic niches with higher value and less competition. By providing solutions to specific patient problems—such as improving adherence through more convenient dosing regimens or offering safer formulations—generic companies can differentiate their products beyond simple price competition. This approach enables them to capture a higher market value and establish a more sustainable competitive position.
The Rise of “Super Generics” and Value-Added Formulations
The generic pharmaceutical industry is evolving beyond the traditional model of simply producing bioequivalent copies. A new wave of “super generics” and value-added formulations is emerging, representing a strategic pivot towards innovation in product design and delivery.
Enhanced Delivery Systems and Patient Convenience
“Higher-value generics” are those that offer improved efficacy, enhanced safety, or greater convenience compared to conventional generic products . This differentiation is often achieved through the incorporation of advanced drug delivery systems. Examples include extended-release formulations, which reduce dosing frequency (e.g., from multiple times a day to once daily) , transdermal patches that offer continuous drug delivery and bypass the digestive system , and sophisticated injectable systems like long-acting injectables (LAIs), nanoparticles, and liposomes . These innovations directly translate into enhanced patient convenience and potentially better adherence, especially for chronic conditions requiring long-term medication. Innovation in formulation and delivery systems becomes a key differentiator beyond mere price, offering enhanced patient benefits and premium positioning. This evolution represents a strategic pivot for the industry, moving beyond the commoditization of simple generics. By investing in R&D to develop these advanced formulations, generic manufacturers can create products that offer tangible improvements in patient experience and clinical outcomes. This allows them to command a premium price and attract a broader customer base, shifting the competitive focus from lowest cost to highest value.
Differentiated Products for Premium Positioning
By mastering advanced formulation technologies, generic manufacturers can develop products with enhanced therapeutic profiles that not only offer patient benefits but also potentially qualify for regulatory pathways that provide periods of limited competition. This strategic approach allows companies to capture additional value commensurate with the associated additional risk and patient benefit, moving beyond the razor-thin margins of simple generics . The growing emphasis on value-based care and patient-centricity is driving demand for “super generics” and biosimilars, creating a market shift where improved outcomes and convenience are rewarded alongside affordability. As healthcare systems globally move towards models that incentivize value over volume, payers and healthcare providers are increasingly recognizing and rewarding products that offer better patient outcomes, improved adherence, and greater convenience. This market shift encourages generic manufacturers to invest in differentiated products that can meet these evolving demands, allowing them to position themselves more strategically and capture higher margins compared to undifferentiated, commoditized generics.
Leveraging Competitive Intelligence and Digital Transformation
In the highly competitive generic pharmaceutical market, success hinges not only on scientific and manufacturing prowess but also on astute business strategy. Leveraging competitive intelligence and embracing digital transformation are becoming indispensable tools for identifying opportunities and optimizing market entry.
Patent Data Analysis for Strategic Decision-Making (e.g., DrugPatentWatch)
For generic developers, precisely timing market entry is paramount. This requires continuous and sophisticated monitoring of patent expiration dates and exclusivity periods for brand-name drugs. Tools and platforms that provide comprehensive patent data analysis and competitive intelligence are invaluable in this regard. For instance, DrugPatentWatch offers critical insights that enable developers to reduce the time required to bring generic drugs to market, thereby increasing their competitiveness. By providing detailed information on patent expiry dates and regulatory timelines, such platforms empower generic companies to anticipate market opportunities, prepare their Abbreviated New Drug Applications (ANDAs) well in advance, and strategically position themselves to achieve the coveted “first-to-file” advantage. In the highly competitive generic market, sophisticated patent data analysis and competitive intelligence are no longer optional but essential tools for identifying lucrative opportunities and timing market entry for maximum impact. This detailed understanding of the intellectual property landscape enables generic firms to accurately predict when a brand-name drug will lose its exclusivity. This foresight is critical for strategic planning, allowing companies to prepare their ANDA submissions and manufacturing capabilities to be ready for the earliest possible market entry, thereby maximizing the potential for the “first-to-file” 180-day exclusivity and its substantial financial rewards.
AI and Machine Learning in Generic Drug Development and Launch
Digital transformation, particularly the adoption of advanced technologies like Artificial Intelligence (AI) and Machine Learning (ML), is poised to revolutionize various aspects of generic drug development and launch strategies. These technologies can streamline drug formulation processes, potentially lowering development costs and expanding patient access by accelerating the availability of new generics .
Beyond development, AI and ML offer powerful predictive capabilities for market analysis. Predictive modeling, for example, can analyze historical launch data to forecast demand, anticipate pricing pressures, and even identify which physicians are most likely to prescribe a new generic . A study by McKinsey found that companies leveraging predictive analytics in drug launches achieved 20% higher market penetration within six months . This technological leap promises unprecedented efficiency, cost reduction, and predictive capabilities across the generic drug lifecycle. By adopting digital technologies, generic manufacturers can significantly improve operational efficiency, lower costs, and enhance customer engagement in an increasingly digital market. This includes optimizing R&D processes, streamlining supply chain management, and creating more targeted and effective marketing campaigns.
Successful Generic Drug Launch Strategies: Case Studies and Best Practices
Achieving success in the generic drug market requires more than just regulatory approval; it demands a comprehensive and meticulously executed launch strategy. This involves a combination of early market entry, targeted marketing, and strategic collaborations.
Early Market Entry and Targeted Marketing
Gaining a competitive edge in the generic market often hinges on early market entry . Being among the first to launch a generic product allows companies to establish brand recognition and capture significant market share before a multitude of competitors emerge and drive prices down. This necessitates thorough market research to pinpoint high-demand therapeutic areas, analyze patient demographics, and understand prescribing patterns . For instance, identifying gaps in generics for chronic conditions like diabetes or cardiovascular drugs can reveal lucrative opportunities .
Once a target is identified, a robust and targeted marketing strategy is essential. This involves crafting a compelling brand identity for the generic product, developing effective marketing materials, and executing well-defined marketing campaigns to boost recognition and drive sales . Digital marketing, including SEO-optimized content, targeted online advertisements, and educational webinars for healthcare professionals, can significantly amplify reach and influence prescribing patterns . For example, a generic launch for atorvastatin reportedly saw a 25% uptake boost after a digital campaign specifically targeting cardiologists . Successful generic launches are not merely about regulatory approval but about meticulous market analysis, precise timing, and aggressive, data-driven commercialization strategies. These elements combine to ensure rapid market penetration. Meticulous market analysis allows companies to understand the competitive landscape and identify unmet needs. Precise timing, often driven by patent intelligence, ensures that the product enters the market at the most opportune moment. Aggressive, data-driven commercialization strategies then ensure that the product quickly gains traction and market share, maximizing its profitability before intense competition sets in.
Strategic Partnerships and Payer Engagement
Building strong relationships and strategic partnerships across the healthcare ecosystem is paramount for maximizing generic drug uptake and ensuring widespread patient access. This includes cultivating relationships with wholesalers, distributors, and healthcare providers .
Engaging with payers, such as Pharmacy Benefit Managers (PBMs) and health plans, is also crucial for securing favorable formulary placement and reimbursement. Presenting data that demonstrates the cost-effectiveness of the generic drug can be instrumental in these negotiations . In 2022, generics that established payer partnerships reportedly achieved 15% higher reimbursement rates .
Case Study: Teva’s Generic Viagra Launch
Teva’s launch of generic Viagra (sildenafil) in 2017 serves as a compelling case study of a successful generic launch. By strategically filing early, pricing aggressively at approximately $35 per pill (compared to $65 for the brand), and targeting urologists with data-driven campaigns, Teva rapidly captured an impressive 70% of the market within a year . This success was attributed to turning market data into a clear roadmap for market domination.
Case Study: Zarxio (Filgrastim-sndz) and Truxima (Rituximab-abbs) Biosimilar Launches
The launch of biosimilars also provides valuable lessons. Zarxio (filgrastim-sndz), introduced by Sandoz in 2015, was the first FDA-approved biosimilar in the U.S. Its success was underpinned by a clear regulatory framework provided by the BPCIA, proven clinical efficacy demonstrating its equivalence to the reference biologic Neupogen, and a significant cost advantage . This launch paved the way for future biosimilars and expanded access to vital treatments for cancer patients. Similarly, Truxima (rituximab-abbs), launched by Celltrion in partnership with Teva Pharmaceuticals in 2019, became the first Rituxan biosimilar approved in the U.S. Its success was driven by strategic partnerships that leveraged Teva’s distribution network and early market entry into the high-demand oncology and immunology spaces . These examples underscore that effective collaboration across the healthcare ecosystem—from distribution partners to payers and prescribers—is paramount for maximizing generic drug uptake and ensuring widespread patient access. Strategic partnerships amplify market reach and secure crucial reimbursement, while payer engagement ensures that the generic product is accessible and affordable within health plans.
The Evolving Landscape: Expert Predictions and Future Trends
The generic drug market is in a constant state of evolution, driven by economic pressures, technological advancements, and shifting healthcare priorities. Expert predictions offer valuable insights into the future trajectory of this critical sector.
Brent Eberle, President of CivicaScript, a company focused on manufacturing and distributing generic medicines at lower prices, offers several key predictions for the industry’s future:
- Risk Mitigation: A continued focus on mitigating risks within the supply chain, especially given that over 90% of prescriptions in the U.S. are generics .
- Fewer Inpatient Generic Drug Shortages: Increased domestic production is expected to reduce the number of active drug shortages, which stood at 270 in 2025 .
- More Competition: Despite current challenges, the market will likely see continued competition.
- Increased Transparency: A significant push towards greater transparency throughout the drug supply chain, from manufacturing costs to patient pricing .
- Eventual Price Stabilization: While generic prices have historically dropped significantly with increased competition, Eberle anticipates that they will eventually reach a floor and stabilize .
The push for increased domestic manufacturing is a particularly strong trend. The movement to bring more drug manufacturing back to the U.S. aims to reduce dependence on overseas suppliers, which can mitigate quality concerns and lead to a more predictable supply and pricing . This strategic shift is a direct response to vulnerabilities exposed in global supply chains. The future of the generic drug market will be defined by a greater emphasis on supply chain resilience, domestic manufacturing, and transparency, shifting the competitive focus beyond pure price to encompass quality, reliability, and patient value. These trends directly address current vulnerabilities, such as drug shortages and quality concerns, and aim to create a more stable and trustworthy generic drug supply. For generic manufacturers, this means that future competitive advantage will increasingly depend on investments in robust manufacturing, transparent operations, and a clear demonstration of value beyond just being the lowest-cost option.
Key Takeaways
The development and commercialization of generic drugs for chronic diseases represent a critical nexus of public health imperative and strategic business opportunity. The escalating global burden of chronic diseases underscores an ever-growing demand for affordable, accessible, and high-quality medications for long-term management. Generic drugs, by offering bioequivalent alternatives at significantly lower costs, are foundational to healthcare affordability and patient adherence, ultimately driving improved health outcomes and systemic cost savings.
However, navigating this landscape requires a nuanced understanding of its inherent complexities. The regulatory pathways, from the streamlined ANDA for small molecules to the intricate 505(b)(2) and 351(k) pathways for complex generics and biosimilars, demand specialized scientific and legal expertise. Innovator companies employ sophisticated intellectual property strategies, including patent layering and evergreening tactics, to delay generic entry, transforming market access into a strategic legal battleground. Despite these challenges, the recurring “patent cliff” offers predictable, high-value opportunities for generic manufacturers, especially those poised to be “first-to-file” and capitalize on temporary market exclusivity.
The sustainability of the generic market hinges on overcoming critical operational challenges. Intense price competition, while beneficial for consumers, can paradoxically undermine manufacturing quality and supply chain resilience, leading to drug shortages. Addressing this necessitates a strategic shift towards investing in advanced manufacturing technologies like continuous processing and promoting domestic production to ensure supply stability. Furthermore, opaque reimbursement policies, particularly those involving Pharmacy Benefit Managers (PBMs), can distort the true cost-saving potential of generics, highlighting the need for greater transparency and policy reforms. Finally, bridging the gap between scientific equivalence and patient/physician perceptions of generics through targeted communication and education is vital for maximizing adoption and adherence.
The future of generic drugs for chronic diseases lies in innovation beyond mere replication. The rise of “super generics” and value-added formulations, offering enhanced delivery systems and patient convenience, represents a key differentiator. Leveraging competitive intelligence, advanced patent data analysis (such as through DrugPatentWatch), and digital transformation technologies like AI and machine learning will be crucial for identifying high-potential therapeutic areas, optimizing development, and executing successful launch strategies. Ultimately, success in this evolving market will depend on a holistic approach that integrates scientific rigor, legal acumen, operational excellence, and a deep commitment to patient value and supply chain resilience.
Frequently Asked Questions (FAQ)
What is the primary difference between a generic drug, a biosimilar, and a complex generic?
A generic drug (typically small molecule) is chemically identical to its brand-name counterpart in its active ingredient and is demonstrated to be bioequivalent, meaning it performs the same in the body. A biosimilar is a “highly similar” version of a complex biological medicine, derived from living systems, and requires comprehensive comparability studies to show no clinically meaningful differences. A complex generic, while also a copy of a small molecule, involves complexities in its active ingredient, formulation (e.g., liposomes, nanoparticles), dosage form, route of administration, or is a drug-device combination product, often requiring more specialized studies beyond standard bioequivalence.
How does the “first-to-file” strategy benefit generic drug manufacturers, and what role does it play in market competition?
The “first-to-file” strategy allows a generic manufacturer to submit an Abbreviated New Drug Application (ANDA) with a Paragraph IV certification, challenging the validity or infringement of a brand-name drug’s patent. If successful, this “first applicant” is granted 180 days of market exclusivity. This temporary monopoly allows the generic company to enter the market with significantly less competition, capture substantial market share, and maintain higher profit margins before other generic versions become available, thereby maximizing their initial return on investment.
What are “evergreening” strategies, and how do they impact the availability of generic drugs for chronic diseases?
“Evergreening” refers to tactics employed by brand-name pharmaceutical companies to extend the patent life of their drugs, thereby delaying generic competition. These strategies often involve patenting minor modifications to the original drug, such as new formulations, dosage forms, or methods of use, or creating “patent thickets” of overlapping patents. These tactics can force generic manufacturers into costly and time-consuming litigation, or delay their market entry, ultimately limiting patient access to more affordable generic alternatives for chronic diseases.
Why are drug shortages more common for generic drugs, and what steps are being taken to address this?
Generic drug shortages are more common due to intense price competition that drives down profit margins, disincentivizing manufacturers from investing in redundant production capacity or maintaining buffer inventories. This often leads to a concentrated supply base, making the supply chain brittle and vulnerable to disruptions. To address this, strategies include diversifying the supply base, promoting domestic manufacturing to reduce reliance on overseas suppliers, and encouraging investments in advanced manufacturing technologies like continuous processing to enhance quality and efficiency.
How do Pharmacy Benefit Managers (PBMs) influence the cost and accessibility of generic drugs for patients with chronic conditions?
PBMs manage prescription drug benefits and design formularies, which determine which drugs are covered and at what cost to the patient. While PBMs aim to control costs, practices like “spread pricing” (charging insurers more than they reimburse pharmacies) and opaque “clawbacks” can inflate the actual cost of generics, leading to higher patient out-of-pocket expenses. This variability in cost-sharing, even for generics, can still create affordability barriers, potentially undermining medication adherence and limiting the full cost-saving potential of generic drugs for patients with chronic conditions.
References
14
24
2 |
Works cited
- Biosimilar medicines: Overview – EMA – European Union, accessed July 25, 2025, https://www.ema.europa.eu/en/human-regulatory-overview/biosimilar-medicines-overview
- The ANDA Process: A Guide to FDA Submission & Approval – Excedr, accessed July 25, 2025, https://www.excedr.com/blog/what-is-abbreviated-new-drug-application
- Differences in the regulatory pathways for biosimilar development: EU vs USA, accessed July 25, 2025, https://resource.ddregpharma.com/blogs/differences-in-the-regulatory-pathways-for-biosimilar-development-eu-vs-usa/
- GENERIC DRUGS IN THE UNITED STATES: POLICIES TO ADDRESS PRICING AND COMPETITION, accessed July 25, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC6355356/
- Generic Drug Access & Savings in the U.S., accessed July 25, 2025, https://accessiblemeds.org/wp-content/uploads/2024/12/2017-AAM-Access-Savings-Report-2017-web2.pdf
- Types of Blood Pressure Medications | American Heart Association, accessed July 25, 2025, https://www.heart.org/en/health-topics/high-blood-pressure/changes-you-can-make-to-manage-high-blood-pressure/types-of-blood-pressure-medications
- 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines, accessed July 25, 2025, https://www.ahajournals.org/doi/10.1161/CIR.0000000000001063
- Clinical Studies for Generic Patches – amarintech.com, accessed July 25, 2025, https://amarintech.com/clinical-studies-for-generic-patches/
- FYs 2013 – 2017 GDUFA Science and Research Report: Transdermal Drug Products | FDA, accessed July 25, 2025, https://www.fda.gov/industry/generic-drug-user-fee-amendments/fys-2013-2017-gdufa-science-and-research-report-transdermal-drug-products
- An expanding horizon of complex injectable products: development and regulatory considerations – PMC – PubMed Central, accessed July 25, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC9376055/
- Foundational Concepts Generics and Biosimilars – FDA, accessed July 25, 2025, https://www.fda.gov/media/154912/download
- Pharmaceutical Patenting – D Young & Co, accessed July 25, 2025, https://www.dyoung.com/en/knowledgebank/articles/pharmaceuticalpatenting0213
- Crystallized Control: Unlocking the Secrets to Polymorph Patents – Kan and Krishme, accessed July 25, 2025, https://kankrishme.com/crystallized-control-unlocking-thesecrets-to-polymorph-patents/
- Facts About Generic Drugs – FDA, accessed July 25, 2025, https://www.fda.gov/media/83670/download
- What Is the Approval Process for Generic Drugs? – FDA, accessed July 25, 2025, https://www.fda.gov/drugs/generic-drugs/what-approval-process-generic-drugs
- 7 FAQs About Generic Drugs | Pfizer, accessed July 25, 2025, https://www.pfizer.com/news/articles/7_faqs_about_generic_drugs
- Biosimilar vs Generic Drugs: Key Differences in Healthcare – Medical Packaging Inc, accessed July 25, 2025, https://medpak.com/biosimilar-vs-generic-drugs/
- Inside the ANDA Approval Process: What Patent Data Can Tell You – DrugPatentWatch, accessed July 25, 2025, https://www.drugpatentwatch.com/blog/inside-the-anda-approval-process-what-patent-data-can-tell-you/
- What Is 505(b)(2)? | Premier Consulting, accessed July 25, 2025, https://premierconsulting.com/resources/what-is-505b2/
- What is the 505(b)(2) Regulatory Pathway? – Allucent, accessed July 25, 2025, https://www.allucent.com/resources/blog/what-505b2
- Biosimilar Product Information – FDA, accessed July 25, 2025, https://www.fda.gov/drugs/biosimilars/biosimilar-product-information
- Bioequivalence – Wikipedia, accessed July 25, 2025, https://en.wikipedia.org/wiki/Bioequivalence
- Bioequivalence: Definition, Testing, FDA Approval Standards – Investopedia, accessed July 25, 2025, https://www.investopedia.com/terms/b/bioequivalence.asp
- Complex Generics: Facts, Figures and Who They Benefit – Teva Pharmaceuticals, accessed July 25, 2025, https://www.tevapharm.com/news-and-media/feature-stories/what-are-complex-generics/
- Complex Generics: Charting a new path – IQVIA, accessed July 25, 2025, https://www.iqvia.com/-/media/library/white-papers/complex-generics-charting-a-new-path.pdf
- Expanding global access to complex generics: The case for regulatory convergence – RAPS, accessed July 25, 2025, https://www.raps.org/news-and-articles/news-articles/2025/2/expanding-global-access-to-complex-generics-the-ca
- Interchangeable Biological Products – FDA, accessed July 25, 2025, https://www.fda.gov/media/151094/download
- Understanding Interchangeable Biosimilars at the Federal and State Levels, accessed July 25, 2025, https://www.ajmc.com/view/understanding-interchangeable-biosimilars-at-the-federal-and-state-levels
- Noncommunicable diseases – World Health Organization (WHO), accessed July 25, 2025, https://www.who.int/health-topics/noncommunicable-diseases
- Noncommunicable diseases – World Health Organization (WHO), accessed July 25, 2025, https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases
- Americans’ Challenges with Health Care Costs – KFF, accessed July 25, 2025, https://www.kff.org/health-costs/issue-brief/americans-challenges-with-health-care-costs/
- Health and Economic Benefits of Chronic Disease Interventions – CDC, accessed July 25, 2025, https://www.cdc.gov/nccdphp/priorities/index.html
- Perceptions of and Barriers to Use of Generic Medications in a Rural African American Population, Alabama, 2011 – CDC, accessed July 25, 2025, https://www.cdc.gov/pcd/issues/2012/12_0010.htm
- Generic Competition and Drug Prices | FDA, accessed July 25, 2025, https://www.fda.gov/about-fda/center-drug-evaluation-and-research-cder/generic-competition-and-drug-prices
- What are the therapeutic areas that receive the most attention in terms of their drug R&D pipeline? – Patsnap Synapse, accessed July 25, 2025, https://synapse.patsnap.com/article/what-are-the-therapeutic-areas-that-receive-the-most-attention-in-terms-of-their-drug-rd-pipeline
- Research and development areas of focus – Bristol Myers Squibb, accessed July 25, 2025, https://www.bms.com/researchers-and-partners/areas-of-focus.html
- U.S. Pharmaceutical Market Size | Industry Report, 2030 – Grand View Research, accessed July 25, 2025, https://www.grandviewresearch.com/industry-analysis/us-pharmaceuticals-market-report
- State of Patient Access scorecard finds that patients with chronic illness continue to face challenges accessing and affording healthcare – PAN Foundation, accessed July 25, 2025, https://www.panfoundation.org/state-of-patient-access-scorecard-finds-that-patients-with-chronic-illness-continue-to-face-challenges-accessing-and-affording-healthcare/
- Generic Drugs Can Help Promote Health Equity – FDA, accessed July 25, 2025, https://www.fda.gov/media/173765/download
- “Skinny Labels” for Generic Drugs Under Hatch-Waxman | Congress.gov, accessed July 25, 2025, https://www.congress.gov/crs-product/IF12700
- Biosimilar Medicines: From Development Process to Marketing Authorization by the EMA and the FDA – MDPI, accessed July 25, 2025, https://www.mdpi.com/2076-3417/14/17/7529
- Regulatory considerations for biosimilar extrapolation of indications – DrugPatentWatch, accessed July 25, 2025, https://www.drugpatentwatch.com/blog/regulatory-considerations-for-biosimilar-extrapolation-of-indications/
- Controversies in Establishing Biosimilarity: Extrapolation of Indications and Global Labeling Practices – PMC, accessed July 25, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC4746210/
- Complex Drug-Device Generic Combination Products Meeting, accessed July 25, 2025, https://www.diaglobal.org/productfiles/8755070/18035%20Program.pdf
- FDA-EMA Parallel Scientific Advice Pilot Program for Complex Generic/Hybrid Drug Products, accessed July 25, 2025, https://www.fda.gov/media/173402/download
- Journal of Chemical Health Risks Regulatory Frameworks Adopted by the US and EU for Bringing Complex Generics in the Market, accessed July 25, 2025, https://jchr.org/index.php/JCHR/article/download/6930/4122/13039
- Beta blockers – Mayo Clinic, accessed July 25, 2025, https://www.mayoclinic.org/diseases-conditions/high-blood-pressure/in-depth/beta-blockers/art-20044522
- High Blood Pressure Medicines Guide – GovInfo, accessed July 25, 2025, https://www.govinfo.gov/content/pkg/GOVPUB-HE20-PURL-gpo154857/pdf/GOVPUB-HE20-PURL-gpo154857.pdf
- The Role of Generic Drug and its Function – Longdom Publishing SL, accessed July 25, 2025, https://www.longdom.org/open-access/the-role-of-generic-drug-and-its-function-103679.html
- What is a generic drug, and how does it get approved? – Patsnap Synapse, accessed July 25, 2025, https://synapse.patsnap.com/article/what-is-a-generic-drug-and-how-does-it-get-approved
- Generic and hybrid applications | European Medicines Agency (EMA), accessed July 25, 2025, https://www.ema.europa.eu/en/human-regulatory-overview/marketing-authorisation/generic-hybrid-medicines/generic-hybrid-applications
- Regulatory Considerations Specific to Liposome Drug Development as Complex Drug Products – Frontiers, accessed July 25, 2025, https://www.frontiersin.org/journals/drug-delivery/articles/10.3389/fddev.2022.901281/full
- 8. What are the challenges for demonstrating that immunogenicity risks for the biosimilar are less or equivalent to the innovator material? – Bioanalysis Zone, accessed July 25, 2025, https://www.bioanalysis-zone.com/8-what-are-the-challenges-for-demonstrating-that-immunogenicity-risks-for-the-biosimilar-are-less-or-equivalent-to-the-innovator-material_ate_biosimilars_charles-river-labs_bioagilityx/
- Biosimilars: Determining Immunogenic Potential | Amgen Oncology, accessed July 25, 2025, https://www.amgenoncology.com/resources/biosimilars-determining-immunogenic-potential-USA-BIO-80007.pdf
- Patents and Exclusivity | FDA, accessed July 25, 2025, https://www.fda.gov/media/92548/download
- Frequently Asked Questions on Patents and Exclusivity – FDA, accessed July 25, 2025, https://www.fda.gov/drugs/development-approval-process-drugs/frequently-asked-questions-patents-and-exclusivity
- Evergreening – Wikipedia, accessed July 25, 2025, https://en.wikipedia.org/wiki/Evergreening
- Evergreening – Definitive Healthcare, accessed July 25, 2025, https://www.definitivehc.com/resources/glossary/evergreening
- Data exclusivity | European Medicines Agency (EMA), accessed July 25, 2025, https://www.ema.europa.eu/en/glossary-terms/data-exclusivity
- Exclusivity and Generic Drugs: What Does It Mean? | FDA, accessed July 25, 2025, https://www.fda.gov/files/drugs/published/Exclusivity-and-Generic-Drugs–What-Does-It-Mean-.pdf
- Patent Certifications and Suitability Petitions – FDA, accessed July 25, 2025, https://www.fda.gov/drugs/abbreviated-new-drug-application-anda/patent-certifications-and-suitability-petitions
- Paragraph IV Explained – ParagraphFour.com, accessed July 25, 2025, https://paragraphfour.com/paragraph-iv-explained/
- FY2018 Regulatory Science Report: Long Acting Injectables and Implants – FDA, accessed July 25, 2025, https://www.fda.gov/media/129010/download
- In-Depth Look at the Differences Between EMA and FDA – Mabion, accessed July 25, 2025, https://www.mabion.eu/science-hub/articles/similar-but-not-the-same-an-in-depth-look-at-the-differences-between-ema-and-fda/
- Mechanistic Modeling of Complex Injectables: Recommendations to Navigate Regulatory Challenges – FDA, accessed July 25, 2025, https://www.fda.gov/media/166586/download
- Drug Patent Watch: Digital Transformation Accelerates Generic Drug Development Timelines. – GeneOnline News, accessed July 25, 2025, https://www.geneonline.com/drug-patent-watch-digital-transformation-accelerates-generic-drug-development-timelines/
- Most Prescribed Medications in 2025 | Prescription Trends – Definitive Healthcare, accessed July 25, 2025, https://www.definitivehc.com/resources/healthcare-insights/top-outpatient-prescription-medications
- The 50 Most Commonly Prescribed Drugs in the United States – NY Requirements Blog, accessed July 25, 2025, https://nyrequirements.com/blog/the-50-most-commonly-prescribed-drugs-in-the-united-states
- Generic Drug Utilization and Spending Among Medicare Part D Enrollees in 2022 – HHS ASPE, accessed July 25, 2025, https://aspe.hhs.gov/sites/default/files/documents/920ec8349c53a362b27e3b10669dafd4/generic-drug-landscape-ib.pdf
- Potential Clinical and Economic Impact of Switching Branded Medications to Generics, accessed July 25, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC5417581/
- Drug Competition Series – Analysis of New Generic Markets Effect of Market Entry on Generic Drug Prices – HHS ASPE, accessed July 25, 2025, https://aspe.hhs.gov/sites/default/files/documents/510e964dc7b7f00763a7f8a1dbc5ae7b/aspe-ib-generic-drugs-competition.pdf
- Potential Impact of the IRA on the Generic Drug Market – Lumanity, accessed July 25, 2025, https://lumanity.com/perspectives/potential-impact-of-the-ira-on-the-generic-drug-market/
- What Pharmacy Benefit Managers Do, and How They Contribute to Drug Spending, accessed July 25, 2025, https://www.commonwealthfund.org/publications/explainer/2025/mar/what-pharmacy-benefit-managers-do-how-they-contribute-drug-spending
- Reimbursement to Pharmacies for Generic Drugs by Medicare Part D Sponsors – PMC, accessed July 25, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC10698681/
- Generic Drug Entry Timeline: Predicting Market Dynamics After Patent Loss, accessed July 25, 2025, https://www.drugpatentwatch.com/blog/generic-drug-entry-timeline-predicting-market-dynamics-after-patent-loss/
- GENERIC DRUG INDUSTRY DYNAMICS David Reiffen formerly Federal Trade Commission currently Department of the Treasury and Michael, accessed July 25, 2025, https://www.ftc.gov/sites/default/files/documents/reports/generic-drug-industry-dynamics/industrydynamicsreiffenwp_0.pdf
- Generic Drug Makers Prioritize Early Market Entry for Competitive Edge: Analysis Reveals Strategies for Success – GeneOnline, accessed July 25, 2025, https://www.geneonline.com/generic-drug-makers-prioritize-early-market-entry-for-competitive-edge-analysis-reveals-strategies-for-success/
- Competing in the Generic Drug Market: Strategies for Success – DrugPatentWatch, accessed July 25, 2025, https://www.drugpatentwatch.com/blog/competing-in-the-generic-drug-market-strategies-for-success/
- The Generic Drug Supply Chain | Association for Accessible Medicines, accessed July 25, 2025, https://accessiblemeds.org/resources/blog/generic-drug-supply-chain/
- Policy Considerations to Prevent Drug Shortages and Mitigate Supply Chain Vulnerabilities in the United States. – HHS ASPE, accessed July 25, 2025, https://aspe.hhs.gov/sites/default/files/documents/3a9df8acf50e7fda2e443f025d51d038/HHS-White-Paper-Preventing-Shortages-Supply-Chain-Vulnerabilities.pdf
- Overcoming Formulation Challenges in Generic Drug Development – DrugPatentWatch, accessed July 25, 2025, https://www.drugpatentwatch.com/blog/overcoming-formulation-challenges-in-generic-drug-development/
- Innovations in Generic Drug Manufacturing and Formulation: Transforming Products and Reducing Costs – DrugPatentWatch, accessed July 25, 2025, https://www.drugpatentwatch.com/blog/innovations-in-generic-drug-manufacturing-and-formulation-transforming-products-and-reducing-costs/
- Industrial Policy To Reduce Prescription Generic Drug Shortages, accessed July 25, 2025, https://www.americanprogress.org/article/industrial-policy-to-reduce-prescription-generic-drug-shortages/
- Policy Considerations to Prevent Drug Shortages and Mitigate Supply Chain Vulnerabilities in the United States – HHS ASPE, accessed July 25, 2025, https://aspe.hhs.gov/reports/preventing-shortages-supply-chain-vulnerabilities
- Current Good Manufacturing Practice (CGMP) Regulations – FDA, accessed July 25, 2025, https://www.fda.gov/drugs/pharmaceutical-quality-resources/current-good-manufacturing-practice-cgmp-regulations
- Expired RFA-FD-14-013: Post-market Surveillance Evaluation of Authorized Generic Drug Products (U01) – NIH Grants and Funding, accessed July 25, 2025, https://grants.nih.gov/grants/guide/rfa-files/RFA-FD-14-013.html
- Development of complex generics: Insights into trends, challenges, and market opportunities, accessed July 25, 2025, https://www.researchgate.net/publication/388140247_Development_of_complex_generics_Insights_into_trends_challenges_and_market_opportunities
- The Global Market For Generics Biosimilars Through 2032 – Outsourced Pharma, accessed July 25, 2025, https://www.outsourcedpharma.com/doc/the-global-market-for-generics-biosimilars-through-2032-0001
- The Impact of Drug Patent Expiration: Financial Implications, Lifecycle Strategies, and Market Transformations – DrugPatentWatch, accessed July 25, 2025, https://www.drugpatentwatch.com/blog/the-impact-of-drug-patent-expiration-financial-implications-lifecycle-strategies-and-market-transformations/
- Generic drugs: Navigating the Patent Cliff: The Rise of Generic Drugs – FasterCapital, accessed July 25, 2025, https://fastercapital.com/content/Generic-drugs–Navigating-the-Patent-Cliff–The-Rise-of-Generic-Drugs.html
- How to Implement a Successful Generic Drug Launch Strategy – DrugPatentWatch – Transform Data into Market Domination, accessed July 25, 2025, https://www.drugpatentwatch.com/blog/how-to-implement-a-successful-generic-drug-launch-strategy/
- Generic Drug Launch Success Tied to Market Analysis, Targeted Marketing Strategies, and Continuous Monitoring, Drug Patent Watch Finds – GeneOnline, accessed July 25, 2025, https://www.geneonline.com/generic-drug-launch-success-tied-to-market-analysis-targeted-marketing-strategies-and-continuous-monitoring-drug-patent-watch-finds/
- The Global Generic Drug Market: Trends, Opportunities, and Challenges – DrugPatentWatch, accessed July 25, 2025, https://www.drugpatentwatch.com/blog/the-global-generic-drug-market-trends-opportunities-and-challenges/
- Overcoming challenges in market access of generic medicines in the European Union | Request PDF – ResearchGate, accessed July 25, 2025, https://www.researchgate.net/publication/239774481_Overcoming_challenges_in_market_access_of_generic_medicines_in_the_European_Union
- Generic Pharmaceuticals & Quality Assurance – WPRX, accessed July 25, 2025, https://www.wprx.com/news/generic-pharmaceuticals-and-quality-assurance
- Analysis of Drug Shortages, 2018-2023 – NCBI Bookshelf, accessed July 25, 2025, https://www.ncbi.nlm.nih.gov/books/NBK611681/
- Generic Drug Shortages: A Case for Continuous Manufacturing – Allied Business Academies, accessed July 25, 2025, https://www.abacademies.org/articles/generic-drug-shortages-a-case-for-continuous-manufacturing-9344.html
- The Future of Generics – UW School of Pharmacy, accessed July 25, 2025, https://pharmacy.wisc.edu/2025/06/11/the-future-of-generics/
- INVESTIGATING GENERICS: America’s Overlooked Drug Crisis – MedShadow Foundation | Independent Health & Wellness Journalism, accessed July 25, 2025, https://medshadow.org/are-generic-drugs-safe/
- Case studies | Enzene Biosciences, accessed July 25, 2025, https://www.enzene.com/why-enzene/case-studies/
- Case Studies on Successful Biosimilar Launches: Transforming Healthcare – Genefic, accessed July 25, 2025, https://genefic.com/case-studies-on-successful-biosimilar-launches-transforming-healthcare/