Diclofenac - Generic Drug Details
✉ Email this page to a colleague
What are the generic drug sources for diclofenac and what is the scope of patent protection?
Diclofenac
is the generic ingredient in sixteen branded drugs marketed by Zyla, Ibsa, Ibsa Inst Bio, Aurobindo Pharma Ltd, Bionpharma, Strides Pharma, Assertio, Alkem Labs Ltd, Annora Pharma, Par Form, Amici, Novartis, Chartwell Rx, Novast Labs, Rk Pharma, Rubicon, Senores Pharms, Sun Pharm Industries, Teva, Umedica, Watson Labs Teva, Actavis Mid Atlantic, Alembic, Amneal, Amneal Pharms, Aurolife Pharma Llc, Cipla, Encube, Glenmark Pharms Ltd, Hikma, Padagis Israel, Perrigo Pharma Intl, Taro, Fougera Pharms, Haleon Us Holdings, Akorn, Altaire Pharms Inc, Bausch And Lomb, Falcon Pharms, Rising, Sandoz, Javelin Pharms Inc, Apotex, Epic Pharma Llc, Lupin Ltd, Lupin Pharms, Novel Labs Inc, Pai Holdings Pharm, Twi Pharms, Watson Labs Inc, Zydus Lifesciences, Horizon, Nuvo Pharms Inc, Actavis Elizabeth, Aurobindo Pharma Usa, Carlsbad, Micro Labs, Pliva, Roxane, Teva Pharms, Unique, Dexcel Ltd, Riconpharma Llc, Vpna, Pfizer, Actavis Labs Fl Inc, Exela Holdings, Yung Shin Pharm, and Zydus Pharms, and is included in ninety-four NDAs. There are thirty-nine patents protecting this compound and one Paragraph IV challenge. Additional information is available in the individual branded drug profile pages.Diclofenac has forty-four patent family members in twenty-three countries.
There are forty-seven drug master file entries for diclofenac. There are four tentative approvals for this compound.
Summary for diclofenac
| International Patents: | 44 |
| US Patents: | 39 |
| Tradenames: | 16 |
| Applicants: | 69 |
| NDAs: | 94 |
| Drug Master File Entries: | 47 |
| Raw Ingredient (Bulk) Api Vendors: | 114 |
| Clinical Trials: | 419 |
| Patent Applications: | 7,122 |
| Formulation / Manufacturing: | see details |
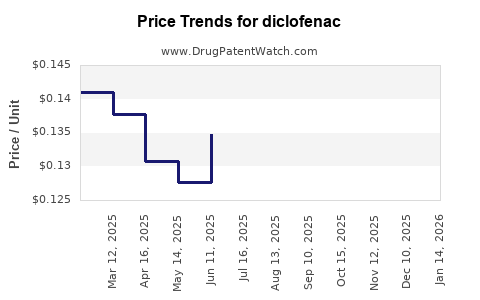
| Drug Prices: | Drug price trends for diclofenac |
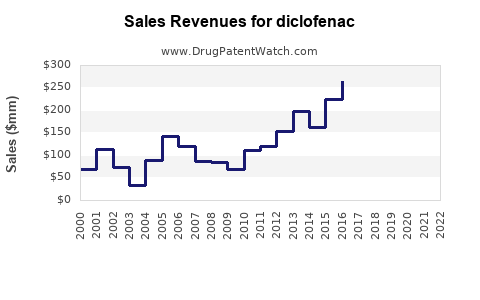
| Drug Sales Revenues: | Drug sales revenues for diclofenac |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for diclofenac |
| What excipients (inactive ingredients) are in diclofenac? | diclofenac excipients list |
| DailyMed Link: | diclofenac at DailyMed |
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for diclofenac
Generic Entry Date for diclofenac*:
Constraining patent/regulatory exclusivity:
Dosage:
CAPSULE;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for diclofenac
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| National Trauma Center | N/A |
| ZetrOZ, Inc. | Phase 1 |
| Daré Bioscience, Inc. | Phase 1 |
Generic filers with tentative approvals for DICLOFENAC
| Applicant | Application No. | Strength | Dosage Form |
| ⤷ Try a Trial | ⤷ Try a Trial | 1.5% W/W | SOLUTION;TOPICAL |
| ⤷ Try a Trial | ⤷ Try a Trial | 2% | SOLUTION;TOPICAL |
| ⤷ Try a Trial | ⤷ Try a Trial | 35MG | CAPSULE;ORAL |
The 'tentative' approval signifies that the product meets all FDA standards for marketing, and, but for the patents / regulatory protections, it would approved.
Medical Subject Heading (MeSH) Categories for diclofenac
Paragraph IV (Patent) Challenges for DICLOFENAC
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| ZORVOLEX | Capsules | diclofenac | 18 mg and 35 mg | 204592 | 1 | 2014-06-06 |
US Patents and Regulatory Information for diclofenac
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Senores Pharms | DICLOFENAC POTASSIUM | diclofenac potassium | TABLET;ORAL | 215787-001 | Mar 15, 2023 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Assertio | ZIPSOR | diclofenac potassium | CAPSULE;ORAL | 022202-001 | Jun 16, 2009 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Aurobindo Pharma Ltd | DICLOFENAC POTASSIUM | diclofenac potassium | CAPSULE;ORAL | 213875-001 | Oct 19, 2021 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Twi Pharms | DICLOFENAC SODIUM | diclofenac sodium | SOLUTION;TOPICAL | 202393-001 | Nov 24, 2014 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Zyla | ZORVOLEX | diclofenac | CAPSULE;ORAL | 204592-002 | Oct 18, 2013 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Horizon | PENNSAID | diclofenac sodium | SOLUTION;TOPICAL | 204623-001 | Jan 16, 2014 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Actavis Elizabeth | DICLOFENAC SODIUM | diclofenac sodium | TABLET, DELAYED RELEASE;ORAL | 074514-001 | Mar 26, 1996 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for diclofenac
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Hong Kong | 1202061 | 雙氯芬酸的新劑型 (A NOVEL FORMULATION OF DICLOFENAC) | ⤷ Try a Trial |
| European Patent Office | 2421525 | FORMULATION DE DICLOFÉNAC (DICLOFENAC FORMULATION) | ⤷ Try a Trial |
| China | 106727424 | 双氯芬酸的新剂型 (A novel formulation of diclofenac) | ⤷ Try a Trial |
| Mexico | 337619 | UNA FORMULACION NOVEDOSA DE DICLOFENACO. (A NOVEL FORMULATION OF DICLOFENAC.) | ⤷ Try a Trial |
| South Korea | 20140124873 | A NOVEL FORMULATION OF DICLOFENAC | ⤷ Try a Trial |
| Australia | 2014208310 | A Novel Formulation of Diclofenac | ⤷ Try a Trial |
| Japan | 6154846 | ⤷ Try a Trial | |
| >Country | >Patent Number | >Title | >Estimated Expiration |