In pharmaceutical innovation time is not just money; it is the very currency of competitive survival. The journey from a promising molecule in a lab to a life-saving therapy on a pharmacy shelf is a decade-long, multi-billion-dollar marathon fraught with scientific uncertainty and regulatory hurdles.1 At the heart of this endeavor lies the patent—not merely a legal document, but the foundational commercial asset that allows an innovator to recoup these staggering investments and fund the next wave of discovery.2 This exclusive right to market an innovation, typically for 20 years from the filing date, is the bedrock of the entire pharmaceutical business model.
However, this 20-year term is a profoundly misleading figure. The patent clock begins ticking the moment an application is filed, often years before a drug candidate even enters human clinical trials. The arduous process of clinical development and regulatory review routinely consumes a massive portion of this term, leaving what is known as the “effective patent life”—the actual period of market exclusivity—at a fraction of the statutory grant, often just 7 to 12 years.2 Every day spent in patent prosecution is a day of potential market exclusivity lost forever. This erosion of commercial runway is the central strategic challenge facing every pharmaceutical and biotechnology company today.
This challenge culminates in the dreaded “patent cliff,” a term that strikes fear into the hearts of pharmaceutical executives. It represents the moment a blockbuster drug’s patent protection expires, opening the floodgates to generic competition and triggering a catastrophic revenue collapse that can reach 80-90% within the first year.4 This is not a distant threat; between 2025 and 2030, analysts project that nearly $236 billion in annual revenue is at risk as dozens of high-revenue products face patent expiration. In this environment, accelerating the time-to-grant for a patent is not a matter of administrative convenience; it is a critical component of lifecycle management and a direct countermeasure to the relentless erosion of a drug’s commercial viability.6
It is against this backdrop of immense pressure and urgency that the Patent Prosecution Highway (PPH) emerges. Far more than a simple administrative shortcut, the PPH is a powerful, global framework designed to streamline and accelerate the patent examination process. By allowing patent offices to share and leverage each other’s work, the PPH offers a strategic pathway for innovators to secure patents faster, enter markets sooner, and build a more robust and defensible global intellectual property fortress.8 This report moves beyond a mere procedural explanation of the PPH. It is an executive-level strategic guide designed to empower business leaders, in-house counsel, and IP portfolio managers to understand, deconstruct, and strategically deploy the PPH. Our objective is to transform your understanding of this system from a tactical tool for saving time into a strategic weapon for turning patent data into a decisive competitive advantage.
Deconstructing the PPH: A Global Network of Accelerated Examination
At its core, the Patent Prosecution Highway is a global work-sharing initiative born from a simple, yet powerful, idea: why should multiple patent offices around the world duplicate the immense effort of searching and examining the same invention filed by the same applicant? This foundational concept has evolved from a modest bilateral experiment into a sophisticated global network that is reshaping the landscape of international patent prosecution.
The Genesis and Evolution of Work-Sharing
The global patent system, for all its importance, has long been plagued by inefficiency. As corporate R&D became increasingly globalized, companies began filing corresponding patent applications for the same core invention in dozens of countries. This created a massive duplication of effort, with examiners in the United States, Europe, Japan, and elsewhere independently conducting prior art searches and substantive examinations on nearly identical sets of claims.10 This redundancy not only created enormous backlogs at major patent offices but also drove up prosecution costs for applicants and, frustratingly, often led to inconsistent examination outcomes across different jurisdictions.8
Recognizing this systemic problem, the Japan Patent Office (JPO) proposed a novel solution. The idea was to create a “highway” for patent applications, allowing the work done by one office to be recognized and utilized by another to speed up the process. This vision became a reality in July 2006 with the launch of a landmark pilot program between the JPO and the United States Patent and Trademark Office (USPTO).13 This initial PPH agreement was a resounding success, demonstrating that work-sharing was not only possible but highly effective.
The success of the USPTO-JPO pilot sparked a rapid global expansion. What began as a single bilateral link quickly grew into a sprawling network of agreements. Coordinated heavily by the JPO, the PPH initiative expanded to include the intellectual property offices (IPOs) of the United Kingdom, Korea, Canada, and Europe within just a few years.15 Today, the PPH network has become a truly global phenomenon, encompassing more than 50 IPOs and serving as a testament to the worldwide acceptance of the work-sharing concept as a cornerstone of modern patent practice.14
Understanding the Core PPH Mechanism
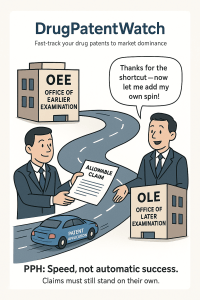
The fundamental principle of the PPH is elegant in its simplicity. It operates on a framework involving two key players: the Office of Earlier Examination (OEE) and the Office of Later Examination (OLE). The process unfolds as follows: an applicant files corresponding patent applications in at least two PPH partner offices. The application is examined first in the OEE. If the examiner in the OEE finds that at least one claim in the application is patentable, the applicant can then leverage this positive result.20 They can file a request with the OLE to have their corresponding application placed on an accelerated examination track.23
It is absolutely critical, however, to understand what the PPH is and what it is not. The PPH is a work-sharing tool designed to promote efficiency; it is not a mechanism for the automatic grant of patents. The OLE retains complete autonomy and is in no way bound by the findings of the OEE.21 The examiner at the OLE will conduct their own substantive examination based on the laws and examination standards of their specific jurisdiction.20 The value of the PPH lies in the fact that the OLE examiner can use the search and examination results from the OEE as a starting point, avoiding the need to duplicate that initial work. This reuse of effort is what drives the acceleration. While statistics consistently show that PPH applications have significantly higher allowance rates and fewer office actions, a grant is never guaranteed, and applicants must be prepared to address new objections raised by the OLE.10
Navigating the PPH Constellation: Types of Agreements
The PPH is not a single, monolithic program but rather a constellation of overlapping agreements and frameworks. Understanding the different types of PPH programs is essential for crafting an effective global filing strategy. Each has its own scope, set of participating offices, and strategic utility.
Bilateral PPH Agreements
Bilateral agreements are the original and most straightforward form of the PPH. They represent a direct, reciprocal work-sharing arrangement between two specific patent offices.15 The pioneering USPTO-JPO program was a bilateral agreement. Over the years, the USPTO and other major offices have established numerous bilateral PPH programs with countries that may not be part of the larger, multilateral frameworks. For example, the USPTO maintains active bilateral PPH pilot programs with offices in France, Malaysia, and Taiwan, among others.27
Strategically, bilateral agreements are most useful for companies with a highly focused IP strategy. If a pharmaceutical company’s primary markets are, for instance, the United States and France, and a strong bilateral PPH agreement exists between them, this direct route can be the most efficient way to accelerate prosecution in the second market.
The Global PPH (GPPH)
As the network of bilateral agreements grew, it became administratively burdensome for applicants to track the slightly different rules and requirements for each country pairing. The Global PPH (GPPH) was created to solve this problem. Launched in 2014, the GPPH is a multilateral framework that establishes a single, harmonized set of rules and procedures for all of its member offices.30 Today, the GPPH includes over 26 patent offices, including major jurisdictions like the USPTO, JPO, UKIPO, CIPO (Canada), and IP Australia.32
The key advantage of the GPPH is its flexibility. Under this framework, a positive examination result from any participating member office can be used as the basis for a PPH request at any other member office.34 This “any-to-any” system is ideal for companies with broad, multi-jurisdictional filing strategies. It allows an applicant to secure an allowance in one GPPH country and then rapidly accelerate examination across a wide swath of other global markets without having to navigate a patchwork of different bilateral rules.
The IP5 PPH Program
The IP5 PPH Program is a specialized plurilateral agreement among the five largest and most influential intellectual property offices in the world: the USPTO, the European Patent Office (EPO), the JPO, the Korean Intellectual Property Office (KIPO), and the China National Intellectual Property Administration (CNIPA).30 This is a powerhouse coalition; collectively, these five offices handle over 90% of the world’s patent applications and represent the most critical commercial markets for the pharmaceutical industry.
The IP5 PPH program allows for streamlined work-sharing among these key jurisdictions.31 For a pharmaceutical or biotech company, this program is of paramount strategic importance. Securing a patent in one of these five major offices can create a powerful domino effect, providing a “golden ticket” to accelerate prosecution in the remaining four. This alignment among the world’s top markets makes the IP5 PPH a cornerstone of any serious global drug patenting strategy.
The PCT-PPH Highway
Perhaps the most significant and powerful evolution of the PPH framework is its integration with the Patent Cooperation Treaty (PCT). The PCT-PPH allows applicants to use a positive work product generated during the international phase of a PCT application as the basis for a PPH request when entering the national phase in participating countries.10 The key documents here are the Written Opinion of the International Searching Authority (WO-ISA) and the International Preliminary Report on Patentability (IPRP).10
The strategic implications of the PCT-PPH are profound. The PCT system allows an applicant to file a single “international” application and delay the costly decision of which specific countries to enter for up to 30 or 31 months from the priority date. During this international phase, the applicant receives a WO-ISA, which provides a preliminary, non-binding opinion on the patentability of the invention. If this opinion is positive, the applicant now holds an incredibly valuable asset. Upon entering the national phase, they can use that single positive report to simultaneously trigger accelerated PPH examination in dozens of countries around the world. This strategy front-loads the substantive examination into the cost-effective PCT phase and provides an early, clear signal of patentability that can be leveraged globally, maximizing both speed and efficiency across an entire patent portfolio.
The complex web of PPH agreements can be daunting, but understanding their distinct strategic advantages allows a company to select the right tool for the right job. The following table provides a concise comparison to aid in this strategic decision-making.
| Program Name | Scope | Key Participating Offices | Primary Basis for Request | Key Strategic Advantage |
| Bilateral PPH | Agreement between two specific offices | USPTO, JPO, DPMA (Germany), INPI (France), etc. | National Work Product from the partner office | Targeted acceleration between two specific, high-value markets. |
| Global PPH (GPPH) | Multilateral agreement with harmonized rules | USPTO, JPO, KIPO, CIPO (Canada), IP Australia, UKIPO, and 20+ others | National or PCT Work Product from any GPPH member | Maximum flexibility for broad, multi-jurisdictional filings under a single set of rules. |
| IP5 PPH | Plurilateral agreement among the five largest IPOs | USPTO, EPO, JPO, KIPO, CNIPA (China) | National or PCT Work Product from any IP5 member | Creates a powerful domino effect across the world’s most critical commercial markets. |
| PCT-PPH | Integration with the Patent Cooperation Treaty | Most PPH-participating offices | Positive PCT Work Product (WO-ISA or IPRP) | Provides an early indication of patentability that can be used to accelerate multiple national entries simultaneously and cost-effectively. |
The evolution of the PPH from simple bilateral pacts to interconnected, multilateral frameworks like the GPPH and IP5, culminating in the integration of the PCT, signifies a fundamental shift in the nature of global patent prosecution. It has created a powerful “network effect.” Previously, a positive result in one country had a linear, one-to-one benefit. Today, that same positive result has an exponential value. It acts as a key that can unlock accelerated examination across a vast, interconnected global network. This transformation elevates the strategic importance of the very first examination. The choice of where to prosecute first is no longer just about securing a patent in that single country; it is a critical decision about which jurisdiction will provide the most potent and widely accepted “golden ticket” to fast-track protection across all other target markets.
The PPH Playbook: A Practical Guide for Pharmaceutical Innovators
Successfully leveraging the Patent Prosecution Highway requires more than just a theoretical understanding; it demands a practical, step-by-step approach to navigating its requirements. This section provides an actionable playbook for pharmaceutical and biotech innovators, focusing on the specific procedures for the two most critical jurisdictions: the USPTO and the EPO.
Step 1: Assessing Eligibility – Is Your Drug Patent a Candidate for the PPH?
Before investing time and resources in preparing a PPH request, the first step is a rigorous eligibility assessment. The process hinges on meeting four core requirements that are largely universal across all PPH programs.
- Relationship Between Applications: There must be a clear link between the application in the Office of Later Examination (OLE) and the application in the Office of Earlier Examination (OEE). This is typically established through a shared priority claim under the Paris Convention or by being part of the same PCT application family.10 This ensures that the two applications are indeed directed to the same invention.
- Allowable Subject Matter: The OEE must have found at least one claim in the corresponding application to be patentable or allowable.10 This “indication of allowability” is the trigger for the entire PPH process. It can come in various forms, including a formal Notice of Allowance, a Decision to Grant, a communication indicating that claims are allowable, or, in some cases, a positive search report. For example, a positive Extended European Search Report (EESR) from the EPO that contains only “A” category citations (documents defining the general state of the art, not prejudicial to novelty or inventive step) against a claim can serve as the basis for a PPH request at the USPTO.22 Similarly, a positive Written Opinion (WO-ISA) or International Preliminary Report on Patentability (IPRP) from the PCT phase is a common and powerful basis for PCT-PPH requests.
- Sufficient Correspondence of Claims: This is the most critical and often most challenging requirement. All claims in the OLE application for which PPH is requested must “sufficiently correspond” to one or more of the claims deemed allowable by the OEE. This means the OLE claims must be of the same, similar, or narrower scope than the OEE’s allowed claims.23 An applicant cannot use the PPH to fast-track an application that contains claims broader than what has already been allowed elsewhere. This requirement imposes a significant strategic constraint, which will be explored in detail later.
- Examination Has Not Begun: The PPH request must be filed at the OLE before substantive examination of that application has commenced.10 The definition of the start of substantive examination varies by office. At the USPTO, for instance, the issuance of a procedural action like a Restriction Requirement does not preclude a subsequent PPH filing. However, once the examiner has issued the first Office Action on the merits of the invention, the window for PPH has closed for that application.19 At the EPO, the rule is similarly strict.25 This makes timing a crucial element of a successful PPH strategy.
It’s also important to note that certain types of applications are explicitly excluded from the PPH program at the USPTO, including provisional applications, plant patents, design patents, and reissue applications.19
Step 2: Preparing the PPH Request Package
Once eligibility is confirmed, the next step is to assemble a precise and complete request package. Incomplete or incorrect submissions are a common reason for PPH requests to be dismissed, causing delays that defeat the purpose of the program. The essential documents typically include:
- The PPH Request Form: Each office has its own specific form. For the USPTO, this is generally Form SB/20 (with variants for different bilateral partners, like SB/20MY for Malaysia) or the global form SB/20GLBL.28 For the EPO, it is Form 1009.51
- OEE Office Actions: A complete copy of all relevant office actions from the OEE that establish the patentability of the claims.18
- The Allowable Claims: A clean copy of the final set of claims that were deemed allowable by the OEE.18
- Information Disclosure Statement (IDS): When filing at the USPTO, an IDS must be submitted listing all the prior art references cited by the OEE examiner during prosecution.
- The Claims Correspondence Table: This is the cornerstone of the application and demands meticulous attention. The applicant must provide a table that maps every single claim in the OLE application to a corresponding allowable claim in the OEE application, with an explanation of how they correspond (e.g., “identical,” “narrower due to the inclusion of feature X,” etc.).18
- Translations: If any of the source documents (office actions, claims) are not in an official language of the OLE, certified translations must be provided.
To ease this administrative burden, many patent offices now participate in digital dossier access systems. Applicants can often simply request that the OLE retrieve the necessary documents directly from systems like the global Dossier Access System or WIPO’s PATENTSCOPE, eliminating the need to manually submit copies.11
Step 3: Filing and Prosecution – A Jurisdiction-Specific Guide
The final step involves filing the request and navigating the subsequent accelerated prosecution. The procedures and strategic considerations can differ significantly between key jurisdictions.
Navigating the USPTO
- Filing: A PPH request at the USPTO must be filed electronically via the Patent Center or EFS-Web. It is crucial to use the correct document description: “Petition to make special under Patent Pros Hwy”.45 This ensures the request is routed correctly and processed promptly.
- Timeline: The benefits in terms of speed are substantial. While standard applications can wait 18-24 months for a first substantive examination, the PPH process is much faster. A decision on the PPH request itself is typically rendered within two to four months, and if granted, the first Office Action on the merits usually follows within another two to three months.12
- Continuation/Divisional Strategy: The USPTO offers a valuable strategic flexibility that is not available in all jurisdictions. If an applicant misses the window to file a PPH request on a parent application (i.e., substantive examination has already begun), they can file a continuation or divisional application. This new application inherits the parent’s priority date but is unexamined, making it eligible for a PPH request based on the allowable claims from the OEE.22 This allows applicants a “second bite at the apple” to get onto the fast track.
Navigating the EPO
- Filing and Requirements: PPH requests at the EPO must be filed using Form 1009. The EPO is particularly strict about the “examination has not begun” requirement, and applicants must verify the status of their application in the European Patent Register before filing.25
- The EPO’s Independent Stance: It cannot be overstated that the EPO takes a rigorous and independent approach to examination. While a granted PPH request will accelerate the procedure under the EPO’s PACE (Programme for Accelerated prosecution of European patent applications), it does not guarantee a favorable outcome. EPO examiners are known for conducting their own thorough searches and are particularly stringent on issues unique to European patent law, such as clarity (Article 84 EPC) and added subject matter (Article 123(2) EPC). It is common for an EPO examiner to raise new objections, even when claims have been allowed by the USPTO or JPO.51
- A Key Tip for Pharma Patents: A crucial detail for life sciences applicants is the EPO’s interpretation of medical use claims. For the purpose of establishing claim correspondence in a PPH request, the EPO considers the older “Swiss-type” claim format (“Use of product X in the manufacture of a medicament for treating Y”) to sufficiently correspond to the modern EPC 2000 second medical use format (“Product X for use in treating Y”). This is a vital piece of practical knowledge that can prevent a PPH request from being rejected on a technicality.
The procedural steps of the PPH may seem straightforward, but they are underpinned by a critical strategic trade-off. The “sufficient correspondence” requirement is not just a box to be ticked; it is a strategic straitjacket that forces an applicant to commit to a specific, and often narrowed, claim scope early in the global prosecution process. Pharmaceutical patent strategy is a long game, often involving the creation of a “patent thicket” with layers of protection, starting with a broad composition of matter patent and later adding narrower patents on specific formulations, dosages, and new methods of use.2 When an applicant uses the PPH, they are effectively locking in the claim scope that was achievable in the very first jurisdiction to grant an allowance. If that first examiner forced a narrowing amendment to overcome prior art, that compromise is now propagated across every subsequent PPH filing. This creates a fundamental tension: the immediate, tangible benefit of speed versus the long-term, strategic risk of being locked into a suboptimal claim scope that may be easier for competitors to design around. Therefore, the decision to enter the PPH must be made not in a vacuum, but with a clear-eyed view of the drug’s entire lifecycle strategy.
The Strategic Calculus: A Data-Driven Analysis of PPH Pros and Cons
The decision to utilize the Patent Prosecution Highway is a strategic one that requires a clear-eyed assessment of its benefits and drawbacks. While the allure of accelerated examination is powerful, it comes with significant trade-offs that must be weighed against a company’s specific commercial goals. This section provides a balanced, data-driven analysis to inform that critical decision.
The Upside: Quantifying the Benefits of the PPH
The advantages of the PPH are not merely anecdotal; they are backed by years of statistical data from the world’s major patent offices. These benefits can be grouped into three main categories: speed, predictability, and cost.
Accelerated Timelines
The most prominent benefit of the PPH is the dramatic reduction in the time it takes to get a patent application examined. This is not a marginal improvement; it is a fundamental shift in the prosecution timeline.
- At the USPTO: For a standard, non-expedited application, the wait for a first substantive office action can be a lengthy 18 to 24 months. In stark contrast, for an application granted PPH status, the average pendency from the petition filing to the first action is approximately 144 days, or just under five months.19 The decision on the PPH request itself is typically made within two months of filing. This effectively shaves more than a year off the front end of the prosecution process.
- At the JPO: The story is similar in other major jurisdictions. At the Japan Patent Office, standard applications wait an average of 9.4 months for a first action. PPH applications receive their first action in an average of just 2.6 to 2.8 months.
For a pharmaceutical company, this acceleration is a powerful strategic lever. It translates directly into earlier certainty about the scope of patent protection, which can de-risk investment decisions, bolster investor confidence, and, most importantly, enable earlier market entry and the establishment of enforceable rights against potential infringers.6
Higher Predictability and Grant Rates
Beyond just being faster, the PPH process is also demonstrably more likely to result in a granted patent.
“According to the cumulative USPTO PPH data for all regions, as of September 30, 2024, a total of 97,397 applications with PPH petitions had been filed from the beginning of the program, and 87,961 of those PPH petition requests were granted. USPTO statistics show that allowance rates are about 10% higher for patents in the PPH program.”
- USPTO Statistics: The data consistently shows a significant uptick in success rates. In fiscal year 2022, the grant rate for PPH applications from major regions was a remarkable 88.3%. In fiscal year 2019, the allowance rate for PPH applications was 84%, compared to an overall rate of 74% for all utility applications.
- First Action Allowances: Perhaps even more impressively, the rate of first action allowances—where an application is granted without any rejections—is notably higher for PPH cases. At the USPTO, this rate was over 30% in FY2022. This means that nearly one in three PPH applications sails through the examination process without any substantive back-and-forth, representing a massive saving in time and resources.
This increased predictability is invaluable for commercial planning. It reduces the uncertainty inherent in the patenting process and strengthens a company’s hand in negotiations for licensing, partnerships, or mergers and acquisitions.7
Cost Efficiency
While the PPH is not primarily a cost-saving tool, it delivers significant financial benefits by streamlining the prosecution process.
- No Official Fees: A major advantage is that most patent offices, including the USPTO and the EPO, do not charge any government fees for filing a PPH request.23 This makes it an accessible option for companies of all sizes.
- Reduced Prosecution Costs: The real savings come from a reduction in legal and administrative costs. Because PPH applications have already been vetted by another office, they tend to face fewer hurdles. Statistics show they receive fewer office actions on average (e.g., 1.5 for PPH cases vs. 1.7 for all cases at the USPTO in FY2019 ), which means fewer attorney hours are spent drafting and filing responses. Furthermore, PPH applications have significantly lower rates of requiring a Request for Continued Examination (RCE) and are less likely to end up in a costly appeal process.16 For a company managing a large global portfolio, these efficiencies can add up to substantial savings over time.8
The Downside: Understanding the Strategic Trade-Offs
The compelling benefits of the PPH are not without their costs. The program imposes significant constraints that can have long-term strategic consequences, creating a classic trade-off between efficiency and flexibility.
The “Sufficient Correspondence” Constraint: A Double-Edged Sword
The single greatest disadvantage of the Patent Prosecution Highway is the strict requirement for “sufficient correspondence” of claims. This is the price of admission to the fast track.
- Loss of Claim Flexibility: The rule that all claims in the OLE application must be of the same, similar, or narrower scope than the allowed OEE claims fundamentally restricts an applicant’s ability to tailor their claiming strategy to the unique legal and commercial landscape of different jurisdictions.12 Patent law is not globally harmonized; a claim that is considered novel and inventive in Europe may be viewed differently in the United States or China. The PPH largely removes the ability to argue for a broader or differently-scoped set of claims in the OLE, forcing a “one-size-fits-all” approach.
- Amendment Restrictions: This constraint extends throughout prosecution. If an OLE examiner raises new prior art that was not considered by the OEE, the applicant’s options for amendment are limited. They must craft an amendment that both overcomes the new rejection and still corresponds to the original OEE claims. This can be extremely difficult, sometimes creating a procedural “dead end” where no compliant amendment is possible. In such cases, the only recourse may be to abandon the PPH application and file a new continuation application to pursue the desired claims, completely negating the time and cost benefits of the PPH.23
The Risk of Suboptimal First Examination
The entire PPH chain is only as strong as its first link: the examination in the Office of Earlier Examination. A company’s global patent protection can become hostage to the outcome of a single negotiation with a single examiner. If, in the interest of securing a quick allowance to enable PPH, a patent prosecutor accepts an overly narrow claim scope from the OEE examiner, that narrow scope will be propagated across every subsequent PPH filing. This can result in a portfolio of quickly-granted but commercially weak patents that are easier for competitors to design around.
The Self-Selection Bias in Statistics
Finally, it is crucial to interpret the impressive PPH success statistics with a degree of analytical rigor. While the numbers are favorable, they are likely influenced by a significant “self-selection bias”. Applications that are eligible for the PPH are, by definition, a pre-screened group. They have already undergone a full examination and been found allowable by a competent patent office. It stands to reason that these applications are, on average, of higher quality and have a stronger intrinsic chance of being allowed than the general pool of unexamined applications. Furthermore, the very act of filing internationally and pursuing an expedited process like the PPH indicates that the applicant believes the invention is commercially important, suggesting they may be more willing to make necessary amendments to secure a patent. This context does not diminish the value of the PPH, but it is essential for setting realistic expectations.
The decision to use the PPH, therefore, exposes a natural tension that can exist within a pharmaceutical organization. The patent prosecution team is often measured on metrics like speed, allowance rate, and cost per patent—all areas where the PPH excels. Their incentive may be to use the program broadly to achieve these goals. However, the commercial, business development, and litigation teams have a different priority: securing the broadest, most robust, and most defensible patent claims possible to maximize market exclusivity and create a powerful tool for enforcement and licensing. The PPH’s core constraint—the loss of claim flexibility—is in direct opposition to this goal. An aggressive, unthinking application of the PPH across a portfolio could lead to a collection of patents that look good on a prosecutor’s scorecard but are less valuable in the marketplace and the courtroom. This highlights the need for the PPH decision to be a cross-functional, strategic one, carefully balancing the immediate procedural benefits against the long-term commercial and legal objectives of the business.
PPH in Action: Tailoring Strategies for Life Sciences Patents
The general principles of the Patent Prosecution Highway apply across all technology sectors, but its strategic application in the life sciences requires a nuanced approach. The unique nature of pharmaceutical and biotechnology inventions, coupled with the complex interplay between patent law and regulatory frameworks, creates specific challenges and opportunities that must be carefully managed.
Strategic Considerations for Small Molecules vs. Biologics
The type of therapeutic modality being protected is a primary factor in determining the optimal PPH strategy. Small molecule drugs and large molecule biologics have fundamentally different patenting landscapes.
- Small Molecule Drugs: For traditional, chemically synthesized drugs, the patent portfolio is often anchored by a “composition of matter” patent covering the active pharmaceutical ingredient (API) itself.62 This is the foundational patent, and securing it quickly and broadly is a top priority. The PPH can be an exceptionally effective tool for this purpose. Once a strong composition of matter claim is allowed in a major jurisdiction like the USPTO or EPO, the PPH can be used to efficiently secure corresponding core patents across the globe.
However, the strategy for small molecules extends far beyond this initial patent. Effective lifecycle management relies on building a “patent thicket” of secondary patents covering different crystalline forms (polymorphs), salts, specific formulations, dosage regimens, and new methods of use. The decision to use PPH for these secondary patents is more complex. For example, patentability standards for polymorphs can differ significantly between the US and Europe. Using PPH might force an applicant to accept a narrow claim scope in one jurisdiction that could have been argued more broadly in another, potentially creating a weak link in the patent thicket. - Biologics (Large Molecules): Biologics, which are complex proteins manufactured in living systems, present an even greater level of patenting complexity.62 The IP protection for a biologic is rarely confined to a single patent. Instead, it is a mosaic of claims covering the amino acid or nucleotide sequence, specific antibodies, the genetically engineered cell lines used for production, complex manufacturing and purification processes, and therapeutic formulations.65
This complexity makes the PPH a riskier proposition. Patentability standards for biologics, particularly concerning enablement and written description requirements, can vary dramatically between major patent offices. A set of claims that the USPTO considers adequately supported might be rejected by the EPO for lacking sufficient data across the entire claimed scope. This divergence increases the difficulty of meeting the “sufficient correspondence” requirement and raises the likelihood that an EPO examiner will disregard the USPTO’s positive finding and conduct a full, independent examination. For biologics, a more cautious, jurisdiction-by-jurisdiction approach may be warranted, reserving the PPH for cases where the allowed claims are based on universally accepted data and principles.
The Critical Link: PPH, Patent Term, and Market Exclusivity
In the pharmaceutical industry, the ultimate value of a patent is determined by its term—the length of time it provides market exclusivity. An expedited prosecution via the PPH can have a direct, and sometimes counterintuitive, impact on this term.
- Patent Term Adjustment (PTA) and Patent Term Extension (PTE):
- PTA: In the United States, the USPTO grants Patent Term Adjustment to compensate for certain delays caused by the patent office during the prosecution of an application. By significantly speeding up the examination process, the PPH can reduce or even eliminate these USPTO-caused delays. This creates a critical trade-off: the applicant gains speed on the front end of prosecution but may forfeit days or months of patent term on the back end that they might have otherwise received as PTA.
- PTE: Patent Term Extension is a separate mechanism that restores a portion of the patent term that was lost due to the lengthy regulatory review process (e.g., clinical trials and FDA approval).56 A company can receive up to five years of PTE. Here, the PPH can be highly advantageous. By ensuring the patent is granted well before the drug is approved, the PPH helps maximize the amount of term eligible for restoration under PTE. For products with very long development timelines, this benefit can be substantial and may outweigh the potential loss of PTA.
- Interplay with Regulatory Exclusivities: It is crucial to distinguish patent protection from regulatory exclusivities granted by agencies like the FDA. These are separate rights that can run concurrently with patents. Key examples include New Chemical Entity (NCE) exclusivity (five years) and Orphan Drug Exclusivity (ODE) (seven years).1 While the PPH does not directly affect these regulatory periods, it plays a vital strategic role. Under the Hatch-Waxman Act, a generic company can challenge a brand’s patents by filing an Abbreviated New Drug Application (ANDA) with a “Paragraph IV” certification. This filing is an act of infringement and often triggers immediate litigation. By using the PPH to ensure that key patents are granted and in force
before the end of the NCE exclusivity period, a pharmaceutical company is in a much stronger position to immediately initiate a patent infringement lawsuit and trigger a 30-month statutory stay of FDA approval for the generic competitor.
Case Study Simulation: Strategic PPH Deployment for a New Cancer Drug
To illustrate these concepts in practice, consider a hypothetical scenario involving “BioGen Inc.,” a biotech company that has developed a promising new antibody for treating a specific type of lung cancer.
- The Scenario: BioGen has strong preclinical data and has just filed a PCT application. The initial Written Opinion (WO-ISA) from the EPO (acting as the International Searching Authority) is highly positive, indicating that the core claims covering the antibody’s sequence and its use in treating lung cancer are novel and inventive. BioGen’s primary commercial markets are the United States, Europe, and Japan. They now face a strategic choice upon national phase entry.
- Strategy 1: The “Speed-to-Portfolio” Approach
BioGen’s immediate goal is to build a portfolio of granted patents as quickly as possible to attract Series B funding and potential licensing partners. They decide to pursue an aggressive PPH strategy.
- Action: Immediately upon entering the national phase in the US, EPO, and JPO, BioGen files PCT-PPH requests in all three jurisdictions, based on the positive WO-ISA. They amend the claims in each application to be identical to the claims found allowable in the PCT report.
- Outcome: The strategy is a resounding success in terms of speed. The USPTO grants a patent in 15 months. The JPO follows suit in 18 months. The EPO, after one office action raising a minor clarity issue, grants a patent in 22 months. BioGen now has a core patent family granted in the three most important global markets in under two years—a powerful story for investors. The trade-off is that the claim scope is locked into what was deemed allowable in the initial, preliminary PCT search.
- Strategy 2: The “Value Maximization” Approach
BioGen is well-funded and its primary goal is to secure the broadest, most defensible patent protection possible to lock down the market for the 10-15 years of its potential commercial life.
- Action: BioGen enters the national phase in all three jurisdictions but does not immediately file for PPH. They choose to pursue standard examination first at the USPTO, their largest and most critical market. Their US attorneys engage in two rounds of negotiation with the USPTO examiner, submitting additional data from ongoing studies to argue for claims that are broader than what was in the original PCT application, covering not just the specific antibody but also related variants with similar functional properties. After three years, they succeed in getting these broader claims allowed.
- Action 2: Now, with a strong, broad US patent in hand, BioGen uses this US allowance as the OEE work product. They file PPH requests at the EPO and JPO, amending the claims in those applications to correspond to the newly allowed, broader US claims.
- Outcome: The overall timeline is longer. The US patent takes three years to grant. The EPO and JPO patents are then granted quickly via PPH within 18 months after the US grant, for a total timeline of about 4.5 years. However, BioGen’s resulting global patent portfolio is significantly more valuable, with a broader scope of protection that is much more difficult for competitors to design around.
- Analysis: This simulation reveals that the “best” PPH strategy is not universal. It is highly dependent on the company’s immediate commercial and financial objectives. For an early-stage company desperate to demonstrate progress and secure funding, the speed of Strategy 1 is paramount.7 The risk of a narrower claim scope is a necessary trade-off for survival. For a more established company focused on maximizing the long-term value of a lead asset, the patient, value-driven approach of Strategy 2 is superior. This demonstrates that the PPH is not a static, one-time decision but a flexible strategic tool that should be deployed in a manner that aligns with the evolving needs of the specific drug program.
Advanced Strategy: Competitive Intelligence and Alternative Pathways
Mastering the Patent Prosecution Highway involves not only understanding its internal mechanics but also knowing how it fits within the broader landscape of patent strategy. This includes comparing it to other acceleration programs and, crucially, leveraging the PPH system as a powerful source of competitive intelligence.
The Acceleration Toolkit: PPH vs. Other Expedited Programs
The PPH is not the only tool available for fast-tracking a patent application. In several key jurisdictions, applicants have other options, each with its own unique set of costs, benefits, and strategic implications. The two most important alternatives for pharmaceutical companies are the USPTO’s Track One program and the EPO’s PACE program.
PPH vs. USPTO Track One Prioritized Examination
- Track One Explained: The Prioritized Examination program, universally known as “Track One,” is the USPTO’s premium offering for speed. For a significant fee (currently $4,200 for a large entity, with reductions for small and micro entities), an applicant can request prioritized examination for a new application. The USPTO’s stated goal for Track One is to reach a final disposition—either an allowance or a final rejection—within an average of 12 months from the date the prioritized status is granted.6
- Head-to-Head Comparison: The choice between PPH and Track One comes down to a classic trade-off: cost versus flexibility.
- Cost: PPH is free. Track One is expensive.
- Eligibility: PPH requires a corresponding application with allowed claims from a partner office. Track One has no such requirement; it can be requested for any new utility or plant application at the time of filing.
- Claim Flexibility: This is the most critical difference. PPH locks the applicant into the claim scope allowed by the OEE. Track One imposes no such restrictions. The applicant is free to pursue claims of any scope and to amend them freely during prosecution (within certain limits on the number of claims).74
- The Synergistic “Track One to PPH” Strategy: The most sophisticated strategy does not view these as mutually exclusive options but combines them for maximum effect. A company can file its initial application in the United States and pay for Track One examination. This allows them to negotiate with the USPTO examiner with full flexibility to achieve the broadest possible claims, and to do so on a highly accelerated timeline (often receiving a first action in just a few months). Once the broad US claims are allowed, the company can then use this quick, high-quality allowance as the basis for filing free PPH requests in all other target countries.6 This powerful one-two punch combines the speed and flexibility of Track One with the global reach and cost-effectiveness of the PPH. It is an expensive initial outlay but can be the most efficient way to build a strong, broad, global patent portfolio in the shortest possible time.
PPH vs. EPO PACE Program
- PACE Explained: The Programme for Accelerated prosecution of European patent applications (PACE) is the EPO’s internal mechanism for speeding up examination. An applicant can file a simple, free request for PACE at any time, which obligates the EPO to issue its next examination report within three months. PACE is less formal than PPH and, crucially, does not impose any restrictions on claim scope.51
- Comparison and Strategic Choice: For an application filed only at the EPO, PACE is almost always the superior choice for simple acceleration due to its greater flexibility and ease of use. The decision becomes more nuanced when a corresponding application exists elsewhere. Filing a PPH request based on an allowed US or JPO patent not only triggers acceleration under the PACE program but also provides the EPO examiner with the persuasive work product from the OEE. While the EPO examiner is not bound by this work, it can serve as a helpful starting point and may streamline the examination process. The strategic choice depends on the applicant’s primary goal: if the aim is simply to speed up the next action without any claim restrictions, PACE is sufficient. If the aim is to accelerate the process and provide the examiner with a favorable precedent from another major office, the PPH is the better tool.
To clarify these strategic choices, the following table provides a direct comparison of the key features of these acceleration programs.
| Key Strategic Factor | Patent Prosecution Highway (PPH) | USPTO Track One | EPO PACE |
| Cost | No official fee | High fee (e.g., $4,200 for large entity) | No official fee |
| Average Time to First Action | ~4-6 months from petition (USPTO) | ~1-2 months from grant of status | ~3 months from request |
| Eligibility Requirement | Corresponding application with allowed claims from a partner office | None (request at filing) | None (request at any time) |
| Impact on Claim Flexibility | High restriction: claims must correspond to allowed OEE claims | No restriction | No restriction |
| Geographic Scope | Global (across all PPH partner offices) | USPTO only | EPO only |
| Best Use Case | Cost-sensitive global filing strategy where claim scope is well-defined and harmonized. | High-value, US-first filing where speed and claim scope flexibility are paramount. | Accelerating a standalone European application without claim restrictions. |
PPH as a Competitive Intelligence Weapon
Beyond its role in accelerating an applicant’s own portfolio, the PPH system is an invaluable and often overlooked source of competitive intelligence. A competitor’s decision to file a PPH request is a public, affirmative act that broadcasts a wealth of strategic information to those who know how to look for it.
- Monitoring Competitor PPH Filings: When a competitor uses the PPH, they are sending a clear signal. They are identifying a specific patent application within their portfolio as being commercially important enough to warrant the effort of fast-tracking. They are also revealing their key target markets—the jurisdictions where they are seeking this accelerated protection. This allows you to filter the “noise” of a large and diverse patent portfolio and focus on the “signal” of what your competitor truly values.
- How to Track PPH Status: While most public patent databases like the EPO’s Espacenet do not have a simple filter to search for “PPH applications,” it is possible to track this activity through diligent monitoring of a competitor’s portfolio. By tracking the legal status and file histories of a competitor’s known patent families across different countries, you can identify the tell-tale signs of a PPH filing: a patent is allowed in one jurisdiction (the OEE), and shortly thereafter, prosecution is rapidly initiated or accelerated in a partner jurisdiction (the OLE).
- Leveraging Specialized Intelligence Platforms: For pharmaceutical companies, a more efficient and powerful approach is to use specialized patent intelligence platforms. Services such as DrugPatentWatch are designed specifically to provide the curated data and analytics needed in the life sciences sector. These platforms monitor global patent and regulatory landscapes, providing alerts on key events like patent litigation, new patent filings, and changes in prosecution status.5 By integrating data from multiple sources, these tools can flag likely PPH filings by competitors and provide the context needed to understand their strategic implications, saving countless hours of manual searching and analysis.83
- Reverse-Engineering Competitor Strategy: By systematically monitoring a competitor’s PPH activity, a company can derive highly actionable intelligence and reverse-engineer their commercial strategy:
- Identifying Key Markets: Where is your competitor focusing their PPH efforts? If they consistently use a US allowance to file PPH requests in Brazil, Canada, and Australia, it provides a clear map of their perceived commercial priorities for that product.
- Pinpointing Core Technology: The patent they choose as the basis for their PPH requests (the OEE application) is likely the one they consider to be the strongest and most fundamental pillar of their IP protection for that product. This allows you to focus your own analysis and potential design-around efforts on their most critical asset.
- Predicting Commercial Timelines: A sudden flurry of PPH filings for a particular drug candidate can be a strong leading indicator of an impending product launch, a major clinical trial data release, or an effort to package the asset for a partnership or acquisition.79 This early warning allows you to adjust your own R&D, patenting, and market-entry strategies accordingly.
In essence, viewing the PPH system through the lens of competitive intelligence transforms it from a simple procedural tool into a real-time window into your competitors’ strategic thinking. It is a powerful way to anticipate their moves and make more informed decisions about your own.
The Road Ahead: The Future of PPH and the Rise of AI
The world of patent prosecution is not static. It is constantly evolving in response to technological advancements, the globalization of innovation, and the ever-present need for greater efficiency. The Patent Prosecution Highway, as a cornerstone of this evolution, continues to develop, while new disruptive forces like Artificial Intelligence are poised to reshape the entire landscape.
The Evolution of Global Work-Sharing
The clear trend in international patent work-sharing is toward deeper integration and greater harmonization. The success of the PPH has created momentum for expanding and simplifying these collaborative efforts.
- Deepening Integration: We are seeing a steady expansion of the major plurilateral agreements. For example, the Brazilian Patent and Trademark Office (BPTO) recently joined the Global PPH, expanding the network and simplifying the process for applicants seeking protection in that key market. There is also a move to make successful pilot programs permanent, providing greater certainty for applicants. The IP5 offices, for instance, have repeatedly extended their PPH pilot and the EPO has made it a permanent program, signaling a long-term commitment to this framework. The ultimate goal is to create a more seamless and user-friendly global prosecution system where the high-quality work of one office can be efficiently leveraged by others.87
- New Models of Collaboration: The success of the PPH has also inspired the development of new and even more ambitious work-sharing models. The USPTO, for example, has piloted programs like the Parallel Patent Grant (PPG) and the Accelerated Patent Grant (APG). Unlike the PPH, which is applicant-driven (the applicant must affirmatively request it), these programs are often office-driven. In the APG, for example, an issued U.S. patent can be used to obtain a grant in a partner country without any further substantive examination, representing an even deeper level of inter-office trust and reliance. These emerging models point to a future where the concept of “one examination, global protection” moves closer to reality.
The Impact of Artificial Intelligence on Patent Prosecution
The most transformative force on the horizon is undoubtedly Artificial Intelligence. AI-powered tools are no longer a futuristic concept; they are actively being deployed and are beginning to revolutionize every stage of the patent prosecution lifecycle.
- AI in Drafting and Prosecution: Sophisticated AI platforms can now assist in drafting patent applications, conducting exhaustive prior art searches in minutes that would take a human hours or days, and even generating shell responses to common types of office action rejections.89 These tools promise to dramatically increase the efficiency of the patenting process, improve the quality of applications by reducing human error, and lower the associated costs.
- AI and the PPH Process: AI has the potential to make the PPH process itself more streamlined and accessible. For instance, AI-powered translation tools can provide fast and increasingly accurate translations of office actions and claims, reducing a significant cost and time barrier for PPH requests. In the near future, AI could be used to automatically analyze the claims of an OEE application and an OLE application and generate a draft claims correspondence table, automating one of the most labor-intensive parts of preparing a PPH request.
- Predictive Analytics: An exciting frontier is the use of AI for predictive analytics. By analyzing vast datasets of past patent applications, examiner statistics, and court decisions, AI tools are beginning to offer predictions on the likelihood of a patent being granted, the probable length of prosecution, and the specific arguments that are most likely to persuade a particular examiner.89 This could allow an applicant to make a far more data-driven decision about whether to use the PPH, and which OEE’s work product is most likely to be favorably received by an OLE.
- Challenges and the Indispensable Human Element: This technological shift is not without its challenges. The use of AI raises significant ethical and legal questions, including data privacy and confidentiality (especially when using third-party cloud-based AI tools), the risk of AI “hallucinations” (generating inaccurate or fictional information), and the ultimate accountability for AI-generated work product.89 The USPTO and other offices are actively developing guidance on the responsible use of AI in practice. This underscores the fact that AI is a tool, not a replacement for human expertise. The nuanced legal judgment, strategic creativity, and deep understanding of a client’s commercial goals remain the indispensable domain of the human patent professional.
As these AI tools become more powerful and ubiquitous, they will begin to commoditize procedural efficiency. The competitive advantage that currently comes from being faster or more efficient at the process of prosecution will diminish, as AI will level the playing field for all participants. In this future landscape, the true source of competitive advantage will shift decisively to the quality of the inputs to that process: the brilliance of the initial invention itself, the sophisticated legal strategy crafted by a seasoned human attorney to protect it, and the high-level business acumen required to decide where in the world that protection is most valuable. The companies that will win in the age of AI-assisted patent prosecution will be those that invest most heavily in world-class R&D and in the strategic, human-led IP counsel needed to guide it.
Conclusion: Integrating the PPH into a Winning Pharmaceutical IP Strategy
The Patent Prosecution Highway is far more than an administrative fast track; it is a powerful and multifaceted strategic tool that, when wielded with skill and foresight, can provide a significant competitive advantage to pharmaceutical and biotechnology innovators. It offers a clear and data-supported pathway to accelerated patent grants, higher allowance rates, and reduced prosecution costs. In an industry where every day of patent life translates directly into commercial revenue, these benefits are not trivial—they are a core component of value creation and lifecycle management.
However, this speed and efficiency come at a price: a significant loss of flexibility. The “sufficient correspondence” requirement acts as a strategic straitjacket, forcing a harmonized claim scope across diverse legal and commercial landscapes. This creates the central strategic dilemma of the PPH: a trade-off between the immediate, tangible benefits of speed and the long-term, strategic value of broad, tailored patent claims.
Navigating this dilemma requires a nuanced, asset-specific approach rather than a one-size-fits-all policy. The optimal strategy is not static but evolves with the commercial and financial objectives of the drug program. Therefore, a clear decision framework is essential:
- Use PPH Aggressively for early-stage assets where building a portfolio of granted patents is critical for attracting investment and partnerships, or for later-stage secondary patents (e.g., new formulations) where the inventive concept is narrow and the claim scope is unlikely to vary significantly between jurisdictions.
- Use PPH Selectively and Patiently for core, high-value assets like a novel composition of matter. For these crown-jewel inventions, the primary goal should be to secure the broadest possible patent protection in the most critical market first. Consider using alternatives like USPTO’s Track One to achieve this with both speed and flexibility, and only then leveraging that strong, broad allowance as the basis for subsequent PPH filings in other territories.
- Always Use PPH for Intelligence. Regardless of whether a company uses the PPH for its own applications, it must incorporate the monitoring of competitor PPH filings into its competitive intelligence workflow. A competitor’s PPH request is one of the clearest available signals of their strategic priorities, key markets, and commercial timelines.
Ultimately, the Patent Prosecution Highway should not be viewed as a standalone tactic but as an integral component of a dynamic and comprehensive global IP strategy. Its successful deployment requires a deep understanding of the specific scientific asset, the competitive and legal landscape in each target market, and the company’s overarching, long-term commercial goals. By mastering the strategic calculus of the PPH, pharmaceutical innovators can more effectively navigate the high-stakes race to market, transforming procedural efficiency into a durable commercial advantage.
Key Takeaways
- PPH is a Revenue-Generating Strategy: By accelerating patent grant, the PPH can extend the effective commercial life of a drug, directly impacting its potential lifetime revenue. It is not just a cost-saving measure.
- Speed vs. Flexibility is the Core Trade-Off: The primary benefit of the PPH is a dramatic reduction in prosecution time. The primary drawback is the loss of claim flexibility due to the “sufficient correspondence” requirement. This trade-off must be evaluated for each specific patent asset.
- The “Track One to PPH” Synergy is a Power Move: For high-value assets, the most powerful strategy can be to use the USPTO’s expensive but flexible Track One program to secure a broad US patent quickly, and then use that allowance as the basis for free, fast PPH filings globally.
- The OEE Choice is a Major Strategic Decision: The choice of which patent office to prosecute in first (the OEE) is critical, as its work product will be the foundation for all subsequent PPH filings. This decision should be based on which office is likely to grant the most valuable and broadly applicable claims.
- PPH is a Competitive Intelligence Goldmine: Monitoring competitors’ PPH filings provides a real-time roadmap of their commercialization strategy, revealing their priority assets and target markets. Platforms like DrugPatentWatch can be instrumental in automating this surveillance.
- Strategy Must Be Asset-Specific: There is no single “best” PPH strategy. An aggressive approach may be right for an early-stage biotech seeking to build a portfolio for investors, while a more patient, value-focused approach is better for a blockbuster drug candidate.
- AI is Changing the Game: Artificial Intelligence is poised to automate many procedural aspects of patent prosecution, including tasks related to PPH filings. This will shift the focus of strategic advantage from procedural mastery to the quality of the underlying invention and the human-led legal and business strategy guiding it.
Frequently Asked Questions (FAQ)
1. What happens if the OEE allows claims, I file a PPH request at the OLE, but then the OEE patent is invalidated in a post-grant proceeding?
This is a complex and jurisdiction-dependent scenario. Generally, the PPH request is based on the examination work product of the OEE at a specific point in time. Once the PPH request is granted by the OLE, the application is placed on the accelerated track. A subsequent invalidation of the OEE patent does not automatically terminate the PPH status at the OLE. However, the applicant would almost certainly have a duty of disclosure to inform the OLE examiner of the invalidation decision and the prior art that formed the basis for it. The OLE examiner would then consider this new information during their substantive examination and could, and likely would, issue a rejection based on it, regardless of the PPH status.
2. Can I use a PPH request based on an allowed formulation patent to expedite examination of an application covering a new method of use for the same drug?
Generally, no. The PPH eligibility requirements are strict regarding the relationship between applications. The OLE application and the OEE application must share a common earliest priority date.20 A patent on a new formulation and a patent on a new method of use are typically considered separate inventions and would likely be filed at different times, with different priority claims. Even if they somehow shared a priority document, the claims themselves would be directed to different statutory classes of invention (a composition vs. a method), making it nearly impossible to demonstrate the “sufficient correspondence” required for a PPH request.
3. My competitor just filed a PPH request in the US based on a Chinese allowance. What are my immediate strategic options to counter this?
This is a valuable piece of competitive intelligence. It signals that your competitor is prioritizing this asset and its US protection. Your options include:
- Analyze the OEE File History: Immediately obtain and analyze the complete prosecution history from the Chinese patent office (the OEE). Understand what prior art was cited and what arguments or amendments were made to get the claims allowed. This is your roadmap to the patent’s potential weaknesses.
- Conduct a Deeper Prior Art Search: The Chinese examiner’s search may not have been exhaustive. Conduct your own targeted search, focusing on US patents and English-language non-patent literature that the original examiner may have missed.
- Consider a Third-Party Pre-Issuance Submission: If your search uncovers strong prior art, you can file a third-party submission at the USPTO to bring this art to the attention of the US examiner before the patent is granted. This can derail the accelerated examination and potentially lead to a rejection.
- Prepare for Post-Grant Challenge: If the patent grants despite your efforts, the analysis you’ve already done will form the basis for a potential post-grant challenge, such as a Post-Grant Review (PGR) or Inter Partes Review (IPR).
4. How does the PPH interact with the new Unified Patent Court (UPC) in Europe? Can a UPC decision be used as a basis for PPH?
This is an emerging area of practice. Currently, PPH programs are based on the work product of patent offices during examination. The UPC is a court that handles litigation for granted European patents. Therefore, a UPC decision on the validity of a patent would not, under the current PPH framework, serve as a basis for a PPH request for an unexamined application. However, the work-sharing principle is evolving. It is conceivable that in the future, patent offices might consider a final, non-appealable validity decision from a respected judicial body like the UPC as persuasive evidence, but this is not part of the PPH system as it exists today.
5. We received a positive PCT Written Opinion, but it included some minor objections under “Box VIII” for clarity. Can we still use this for a PCT-PPH request?
This can be a disqualifying issue. Many patent offices, in their PCT-PPH guidelines, state that if any observations are made in Box VIII of the Written Opinion (“Certain observations on the international application”), the application is not eligible for the PCT-PPH pilot program. These objections, even if they seem minor (like clarity issues or antecedent basis errors), can render the work product “unclean” for PPH purposes. The best strategy in this situation is to address these objections during the international phase by filing a Demand for International Preliminary Examination under Chapter II of the PCT. By filing amendments and arguments, you can work with the International Preliminary Examining Authority (IPEA) to resolve the objections and obtain a clean International Preliminary Report on Patentability (IPRP), which can then be used as a solid basis for PCT-PPH requests.
References
- Patent vs Exclusivity for Pharmaceutical Industry – EXCELON IP, accessed August 6, 2025, https://excelonip.com/patent-vs-exclusivity-for-pharmaceutical-industry/
- Optimizing Your Drug Patent Strategy: A Comprehensive Guide for Pharmaceutical Companies – DrugPatentWatch, accessed August 6, 2025, https://www.drugpatentwatch.com/blog/optimizing-your-drug-patent-strategy-a-comprehensive-guide-for-pharmaceutical-companies/
- How to own a Market you Don’t Own: Market Access Strategies Post-Patent Expiration, accessed August 6, 2025, https://www.drugpatentwatch.com/blog/how-to-own-a-market-you-dont-own-market-access-strategies-post-patent-expiration/
- The End of Exclusivity: Navigating the Drug Patent Cliff for Competitive Advantage – DrugPatentWatch – Transform Data into Market Domination, accessed August 6, 2025, https://www.drugpatentwatch.com/blog/the-impact-of-drug-patent-expiration-financial-implications-lifecycle-strategies-and-market-transformations/
- Patent Defense Isn’t a Legal Problem. It’s a Strategy Problem. Patent Defense Tactics That Every Pharma Company Needs – DrugPatentWatch, accessed August 6, 2025, https://www.drugpatentwatch.com/blog/patent-defense-isnt-a-legal-problem-its-a-strategy-problem-patent-defense-tactics-that-every-pharma-company-needs/
- Expedited Patent Examination: Understanding Your Options in the US and Abroad, accessed August 6, 2025, https://www.foxrothschild.com/publications/expedited-patent-examination-understanding-your-options-in-the-us-and-abroad
- The Impact of PPH on Patent Grant Timelines: What CEOs Should Know – PatentPC, accessed August 6, 2025, https://patentpc.com/blog/the-impact-of-pph-on-patent-grant-timelines-what-ceos-should-know
- Using Patent Prosecution Highway (PPH) to Fast-Track International Filings – PatentPC, accessed August 6, 2025, https://patentpc.com/blog/using-patent-prosecution-highway-pph-to-fast-track-international-filings
- How to Use the Patent Prosecution Highway (PPH) Program – PatentPC, accessed August 6, 2025, https://patentpc.com/blog/how-to-use-the-patent-prosecution-highway-pph-program
- Patent Prosecution Highway – Mewburn Ellis, accessed August 6, 2025, https://www.mewburn.com/law-practice-library/patent-prosecution-highway
- JPO Experience in work-sharing including – Patent Prosecution Highway (PPH) – WIPO, accessed August 6, 2025, https://www.wipo.int/edocs/mdocs/globalinfra/en/wipo_aspec_asean_ip_sin_14/wipo_aspec_asean_ip_sin_14_presentation_3_3.pdf
- Patent Prosecution Highway (PPH) – Fast Track Examination of Patent Applications – Copperpod IP, accessed August 6, 2025, https://www.copperpodip.com/post/patent-prosecution-highway-pph-fast-track-examination-of-patent-applications
- Patent Prosecution Highway (PPH) Program – Federal Register, accessed August 6, 2025, https://www.federalregister.gov/documents/2011/07/08/2011-17077/patent-prosecution-highway-pph-program
- Patent Prosecution Highway (PPH) Snapshot – Sterne Kessler, accessed August 6, 2025, https://www.sternekessler.com/news-insights/insights/patent-prosecution-highway-pph-snapshot/
- A practical guide to the patent prosecution highway | Managing Intellectual Property, accessed August 6, 2025, https://www.managingip.com/Article/2167622/A-practical-guide-to-the-patent-prosecution-highway.html
- Patent Prosecution Highway: The JPO and USPTO – Sterne Kessler, accessed August 6, 2025, https://www.sternekessler.com/news-insights/insights/patent-prosecution-highway-jpo-and-uspto/
- JPOʼs experience in Patent Prosecution Highway – WIPO, accessed August 6, 2025, https://www.wipo.int/edocs/mdocs/pct/en/pct_wg_17/pct_wg_17_presentation_pph_japan.pdf
- Initiatives for Work Sharing (PPH) – WIPO, accessed August 6, 2025, https://www.wipo.int/edocs/mdocs/pct/en/wipo_pct_pre_20/wipo_pct_pre_20_t11.pdf
- Speed Up Patent Prosecution? Think PPH | Finnegan | Leading IP+ Law Firm, accessed August 6, 2025, https://www.finnegan.com/en/insights/blogs/prosecution-first/speed-up-patent-prosecution-think-pph.html
- GENERAL PPH – USPTO, accessed August 6, 2025, https://www.uspto.gov/sites/default/files/documents/FAQs%20-%20Global%20PPH%20-%2005212015.pdf
- Patent Prosecution Highway (PPH) – WIPO, accessed August 6, 2025, https://www.wipo.int/documents/d/scp/docs-expedited-examination-pph.pdf
- GENERAL PPH – USPTO, accessed August 6, 2025, https://www.uspto.gov/sites/default/files/documents/Global%20PPH%20FAQs%20-%20082815_updated.pdf
- Efficiency v Flexibility – The Patent Prosecution Highway (PPH) Program’s Pros and Cons, accessed August 6, 2025, https://www.crowell.com/en/insights/publications/efficiency-v-flexibility-the-patent-prosecution-highway-pph-programs-pros-and-cons-publication
- How does the Patent Prosecution Highway work? – BlueIron IP, accessed August 6, 2025, https://blueironip.com/ufaqs/how-does-the-patent-prosecution-highway-work/
- Patent Prosecution Highway (PPH) | epo.org, accessed August 6, 2025, https://www.epo.org/en/applying/international/patent-prosecution-highway
- The Patent Prosecution Highway (PPH) System – Hlk-ip.com, accessed August 6, 2025, https://www.hlk-ip.com/knowledge-hub/the-patent-prosecution-highway-pph-system/
- Patent Prosecution Highway Between the USPTO and the INPI (Pilot), accessed August 6, 2025, https://www.uspto.gov/patents/basics/international-protection/patent-prosecution-highway/patent-prosecution-highway
- Patent Prosecution Highway (PPH) – Fast Track Examination of …, accessed August 6, 2025, https://www.uspto.gov/patents/basics/international-protection/patent-prosecution-highway-pph-fast-track
- Request for Participation in the Patent Prosecution Highway (PPH) Pilot Program Between the National Patent Office of Bahrain (N – USPTO, accessed August 6, 2025, https://www.uspto.gov/sites/default/files/documents/sb0020my.pdf
- Implementation of the Global and IPS Patent Prosecution Highway (PPH) Pilot Programs with Participating Offices I. Background Be – USPTO, accessed August 6, 2025, https://www.uspto.gov/sites/default/files/documents/global-ip5.pdf
- Shortcuts on the Patent Prosecution Highway – K&L Gates, accessed August 6, 2025, https://files.klgates.com/files/publication/11380a27-1121-4e1c-9681-5ca57f5e1231/presentation/publicationattachment/4de99d7b-6793-4ac6-b22b-6fa55ac7e4d0/ip_alert_02262014.pdf
- Examination under a patent prosecution highway – IP Australia, accessed August 6, 2025, https://www.ipaustralia.gov.au/international-ip/international-cooperation/the-patent-cooperation-treaty/the-patent-prosecution-highway
- Patent Prosecution Highway – DPMA, accessed August 6, 2025, https://www.dpma.de/english/patents/protection_outside_germany/pph/index.html
- Global Patent Prosecution Highway | Intellectual Property Office of New Zealand, accessed August 6, 2025, https://www.iponz.govt.nz/get-ip/patents/apply/expedited-examination-for-patent-applications/global-patent-prosecution-highway/
- PCT-Patent Prosecution Highway Program (PCT-PPH and Global PPH) – WIPO, accessed August 6, 2025, https://www.wipo.int/en/web/pct-system/filing/pct_pph
- Global PPH | Hungarian Intellectual Property Office, accessed August 6, 2025, https://www.sztnh.gov.hu/en/patent/global-pph
- Which patent offices maintain PPH programmes with the EPO? | epo.org, accessed August 6, 2025, https://www.epo.org/en/service-support/faq/applying-patent/patent-prosecution-highway-pph/general-introduction-pph/which
- IP5 Patent Prosecution Highway (IP5 PPH) – fiveIPoffices, accessed August 6, 2025, https://www.fiveipoffices.org/activities/ws/ip5pph
- IP5 PPH Pilot Program | Articles | Finnegan | Leading IP+ Law Firm, accessed August 6, 2025, https://www.finnegan.com/en/insights/articles/ip5-pph-pilot-program.html
- Patent Prosecution Highway – Wikipedia, accessed August 6, 2025, https://en.wikipedia.org/wiki/Patent_Prosecution_Highway
- Patent Prosecution Highway (PPH) and the PCT – WIPO, accessed August 6, 2025, https://www.wipo.int/edocs/mdocs/pct/en/pct_wg_17/pct_wg_17_presentation_pph_international_bureau.pdf
- Expedited examination Patent Prosecution Highway, accessed August 6, 2025, https://ised-isde.canada.ca/site/canadian-intellectual-property-office/en/expedited-examination-patent-prosecution-highway
- New Patent Prosecution Highway Pilot Program Between United States And European Patent Offices – Special Report, accessed August 6, 2025, https://www.oliff.com/wp-content/uploads/2020/11/US-EPO-PPH-Pilot-Program.pdf
- Patent Prosecution Highway (PPH) in EPO – IP-Coster, accessed August 6, 2025, https://www.ip-coster.com/gateway/pph-epo
- General PPH Filing a PPH Request – USPTO, accessed August 6, 2025, https://www.uspto.gov/sites/default/files/documents/FAQs-for-PPH-revised-05032023.pdf
- European Patent Office Patent Prosecution Highway | Intellectual Property Office of New Zealand, accessed August 6, 2025, https://www.iponz.govt.nz/get-ip/patents/apply/expedited-examination-for-patent-applications/european-patent-office-patent-prosecution-highway/
- Patent Prosecution Highway (PPH) in USA – IP-Coster, accessed August 6, 2025, https://www.ip-coster.com/gateway/pph-uspto
- What is PPH (Patent Prosecution Highway)? – Patent Trademark Blog | IP Q&A, accessed August 6, 2025, https://www.patenttrademarkblog.com/what-is-pph/
- 4.3 Patent Prosecution Highway (PPH), accessed August 6, 2025, https://www.epo.org/en/legal/guidelines-epc/2024/e_viii_4_3.html
- Patent Prosecution Highway Between USPTO and DPMA (Pilot), accessed August 6, 2025, https://www.uspto.gov/patents/basics/international-protection/patent-prosecution-highway/patent-prosecution-5
- Requesting Patent Prosecution Highway (PPH) in the EPO or UK IPO – J A Kemp, accessed August 6, 2025, https://www.jakemp.com/knowledge-hub/requesting-patent-prosecution-highway-pph-in-the-epo-or-uk-ipo/
- Exploring the Patent Prosecution Highway (PPH) at the EPO – PCT Suite, accessed August 6, 2025, https://pctsuite.com/exploring-the-patent-prosecution-highway-pph-at-the-epo/
- Japant Patent Office – IPOPHL, accessed August 6, 2025, https://www.ipophil.gov.ph/patent-prosecution-highway-pph/japant-patent-office/
- Patent Prosecution Highway (PPH) – USPTO, accessed August 6, 2025, https://www.uspto.gov/patents/apply/petitions/26-patent-prosecution-highway-pph
- Accelerating Prosecution at the EPO | Finnegan | Leading IP+ Law Firm, accessed August 6, 2025, https://www.finnegan.com/en/insights/blogs/european-ip-blog/accelerating-prosecution-at-the-epo.html
- Effective Drug Patent Prosecution Strategies: Securing Your Pharmaceutical Innovations, accessed August 6, 2025, https://www.drugpatentwatch.com/blog/effective-drug-patent-prosecution-strategies-securing-your-pharmaceutical-innovations/
- FY 2022 PPH STATISTICS DATA – USPTO, accessed August 6, 2025, https://www.uspto.gov/sites/default/files/documents/PPHQuarterlyStatisticsDataFY2021.pdf
- The PPH Program at the USPTO: Favorable Stats Don’t Alleviate Big Risks, accessed August 6, 2025, https://ipwatchdog.com/2019/10/30/pph-program-uspto-favorable-stats-dont-alleviate-big-risks/id=115477/
- Accelerating Global Patent Protection: Role of Patent Prosecution Highway (PPH) & AI, accessed August 6, 2025, https://sagaciousresearch.com/blog/role-of-patent-prosecution-highway-in-accelerating-global-patents/
- Should You Get On The Patent Prosecution Highway? – Marshall, Gerstein & Borun LLP, accessed August 6, 2025, https://www.marshallip.com/content/uploads/2023/07/MG_Kriegel-in-Law360_07-21-23.pdf
- Strategic Use of the Patent Prosecution Highway | Sterne Kessler, accessed August 6, 2025, https://www.sternekessler.com/news-insights/insights/strategic-use-patent-prosecution-highway/
- Difference Between Small Molecule and Large Molecule Drugs – Caris Life Sciences, accessed August 6, 2025, https://www.carislifesciences.com/difference-between-small-molecule-and-large-molecule-drugs/
- A RESPONSE TO THE UN’S GUIDELINES FOR PHARMACEUTICAL PATENT EXAMINATION – IU Indianapolis, accessed August 6, 2025, https://journals.indianapolis.iu.edu/index.php/inlawrev/article/download/21522/20754/0
- Biologic Drugs for Arthritis, accessed August 6, 2025, https://www.arthritis.org/drug-guide/biologics/biologics
- Trade secrets vs patents in life sciences: Striking the right balance – Griffith Hack, accessed August 6, 2025, https://www.griffithhack.com/insights/publications/trade-secrets-vs-patents-in-life-sciences-striking-the-right-balance/
- Biotechnology & Life Sciences Patent Prosecution – Polsinelli, accessed August 6, 2025, https://www.polsinelli.com/biotechnology-life-sciences-patent-prosecution
- How to make the most of the Patent Prosecution Highway – Greaves Brewster, accessed August 6, 2025, https://greavesbrewster.co.uk/how-to-make-the-most-of-the-patent-prosecution-highway/
- Protection beyond 20 years: data on SPCs and other term extensions for pharmaceutical patents | epo.org, accessed August 6, 2025, https://www.epo.org/en/searching-for-patents/helpful-resources/patent-knowledge-news/protection-beyond-20-years-data-spcs
- Exclusivity and Generic Drugs: What Does It Mean? | FDA, accessed August 6, 2025, https://www.fda.gov/files/drugs/published/Exclusivity-and-Generic-Drugs–What-Does-It-Mean-.pdf
- Frequently Asked Questions on Patents and Exclusivity – FDA, accessed August 6, 2025, https://www.fda.gov/drugs/development-approval-process-drugs/frequently-asked-questions-patents-and-exclusivity
- The Role of Patents and Regulatory Exclusivities in Drug Pricing | Congress.gov, accessed August 6, 2025, https://www.congress.gov/crs-product/R46679
- How the PPH Benefits Emerging Technology Patents in Global Markets | PatentPC, accessed August 6, 2025, https://patentpc.com/blog/how-the-pph-benefits-emerging-technology-patents-in-global-markets
- Using Prioritized Examination with PPH for Lightning-Fast Approvals – PatentPC, accessed August 6, 2025, https://patentpc.com/blog/using-prioritized-examination-with-pph-for-lightning-fast-approvals
- What to Do Now That the USPTO Accelerated Examination Program Is Ending, accessed August 6, 2025, https://www.mccarter.com/insights/what-to-do-now-that-the-uspto-accelerated-examination-program-is-ending/
- USPTO’s Track One vs Patent Prosecution Highway – BlueIron IP, accessed August 6, 2025, https://blueironip.com/usptos-track-one-vs-patent-prosecution-highway/
- Accelerated Prosecution at the EPO – Hlk-ip.com, accessed August 6, 2025, https://www.hlk-ip.com/knowledge-hub/accelerated-prosecution-at-the-epo/
- PPH and other acceleration programmes or ways to expedite the grant | epo.org, accessed August 6, 2025, https://www.epo.org/en/service-support/faq/applying-patent/patent-prosecution-highway-pph/pph-and-other-acceleration
- 3 Reasons Companies Should Monitor Competitors’ Patent Filings – LexisNexis IP, accessed August 6, 2025, https://www.lexisnexisip.com/resources/3-reasons-companies-should-monitor-competitors-patent-filings/
- How to Use Patent Monitoring to Track Competitors and Their Patent Filing – Sagacious IP, accessed August 6, 2025, https://sagaciousresearch.com/blog/how-to-use-patent-monitoring-to-track-competitors-and-their-patent-filing/
- Patent Search: Competitors and Own Company – PatCheck, accessed August 6, 2025, https://patcheck.de/learn/zu-welchen-themen-sind-die-patente-angemeldet-worden/
- Drug Patent Watch, accessed August 6, 2025, https://www.drugpatentwatch.com/
- Patent Monitoring During Prosecution and Litigation – Sagacious Research, accessed August 6, 2025, https://sagaciousresearch.com/blog/patent-monitoring-during-prosecution-litigation/
- ARK Patent Intelligence – IQVIA, accessed August 6, 2025, https://www.iqvia.com/solutions/industry-segments/generics/ark-patent-intelligence
- Patent Search Services – Patent Validity Search – Clarivate, accessed August 6, 2025, https://clarivate.com/intellectual-property/patent-intelligence/patent-search-services/
- How to Track Competitors and Their Patent Filings Using Patent Monitoring – XLSCOUT, accessed August 6, 2025, https://xlscout.ai/how-to-track-competitors-and-their-patent-filings-using-patent-monitoring/
- Upcoming Changes to Patent Prosecution Highway Processing in Brazil – IPWatchdog.com, accessed August 6, 2025, https://ipwatchdog.com/2024/10/16/upcoming-changes-patent-prosecution-highway-processing-brazil/id=182100/
- Patent worksharing | USPTO, accessed August 6, 2025, https://www.uspto.gov/ip-policy/patent-policy/patent-worksharing
- The Future of Patent Office Procedures: Trends and Innovations | PatentPC, accessed August 6, 2025, https://patentpc.com/blog/the-future-of-patent-office-procedures-trends-and-innovations
- The Double-Edged Sword of AI in Patent Drafting and Prosecution | AI Law and Policy, accessed August 6, 2025, https://www.ailawandpolicy.com/2024/10/the-double-edged-sword-of-ai-in-patent-drafting-and-prosecution/
- Automation & Predictive Analytics in Patent Prosecution: USPTO Implication & Policy – The Reading Room – Georgia State University, accessed August 6, 2025, https://readingroom.law.gsu.edu/cgi/viewcontent.cgi?article=2978&context=gsulr
- AI in Patent Prosecution: Enhancing Filing a Patent, accessed August 6, 2025, https://sagaciousresearch.com/blog/ai-in-patent-prosecution-the-new-frontier-in-filing-a-patent-and-improving-processes/
- Leveraging AI for More Effective Patent Prosecution Strategies – XLSCOUT, accessed August 6, 2025, https://xlscout.ai/leveraging-ai-for-more-effective-patent-prosecution-strategies/
- Guidance on Use of Artificial Intelligence-Based Tools in Practice Before the United States Patent and Trademark Office – Federal Register, accessed August 6, 2025, https://www.federalregister.gov/documents/2024/04/11/2024-07629/guidance-on-use-of-artificial-intelligence-based-tools-in-practice-before-the-united-states-patent
- How to file a PPH request with SIPO – Managing Intellectual Property, accessed August 6, 2025, https://www.managingip.com/article/2a5bqo2drurt0bwsoikk9/how-to-file-a-pph-request-with-sipo