Introduction: The Imperative for a New Trust Architecture
The global pharmaceutical industry stands at a paradoxical juncture. On one hand, it represents the zenith of human scientific achievement, delivering life-saving therapies and pushing the boundaries of medicine. On the other, its operational foundations are beset by systemic vulnerabilities that erode value, endanger patients, and undermine the very trust upon which the entire healthcare ecosystem is built. This is the pharmaceutical paradox: an industry of profound innovation operating on an infrastructure that often remains archaic, fragmented, and dangerously opaque.
This report will dissect this critical trust deficit by examining three core crises that define the industry’s operational landscape. The first is a shadow pandemic of counterfeit drugs, a global criminal enterprise with a market value estimated to be as high as $431 billion annually, which poses a direct and lethal threat to public health.1 The second is the labyrinthine global supply chain, a system so riddled with inefficiencies that it loses over 7% of its stock to waste and spoilage while simultaneously failing to prevent chronic drug shortages that disrupt patient care.2 The third is a crisis of credibility in clinical research, where persistent data integrity issues can delay the approval of new medicines and cast doubt on the validity of scientific findings.4
These are not isolated problems but deeply interconnected symptoms of a single, fundamental flaw: the absence of a shared, immutable, and verifiable source of truth across a sprawling ecosystem of competing and often mistrustful stakeholders. In response to this systemic challenge, this report posits that blockchain technology is not merely an incremental upgrade or a niche solution, but a foundational “trust layer” capable of re-architecting the industry’s core processes. Through a detailed analysis of real-world applications, pioneering case studies, and strategic intellectual property landscapes, this report will demonstrate how blockchain can forge a more secure, efficient, and transparent pharmaceutical ecosystem. It will ultimately argue that for forward-thinking organizations, the strategic adoption of this technology is not just a matter of operational improvement, but a pathway to generating significant and sustainable competitive advantage.
Part I: The Anatomy of a Trust Deficit: Quantifying the Pharma Industry’s Core Challenges
To fully appreciate the transformative potential of blockchain, it is essential to first quantify the scale and scope of the problems it aims to solve. The pharmaceutical industry’s operational vulnerabilities are not theoretical; they are measurable crises with profound human and economic consequences. This section will dissect the three primary manifestations of the industry’s trust deficit: the proliferation of counterfeit drugs, the systemic inefficiencies of the supply chain, and the persistent challenges to data integrity in clinical research.
1.1 The Shadow Pandemic: The Global Scourge of Counterfeit Drugs
The trade in falsified and substandard medical products represents one of the most significant threats to global public health and economic stability. It is a lucrative, sophisticated, and deadly criminal enterprise that thrives in the shadows of the legitimate pharmaceutical supply chain.
Scope and Scale
The financial magnitude of the counterfeit drug market is staggering. According to the World Health Organization (WHO), the global trade in counterfeit pharmaceuticals is estimated to be worth between $200 billion and $431 billion annually.1 Industry estimates place the figure in a similar range of €150 billion to €200 billion ($163 billion to $217 billion) per year, making counterfeit pharmaceuticals the single most lucrative sector of the global trade in illegally copied goods.6 To put this in perspective, this value is only slightly less than the estimated $246 billion illicit drug trade, and it can be ten times more profitable than trafficking heroin, highlighting the immense financial incentive for criminal organizations.7 The Organization for Economic Co-operation and Development (OECD) estimates that counterfeit goods, including pharmaceuticals, account for 3.3% of all global trade.1
The Human Cost
Beyond the financial figures lies a devastating human toll. The WHO estimates that at least 1 in 10 medicines circulating in low- and middle-income countries are substandard or falsified.8 These products may contain no active ingredient, the wrong active ingredient, an incorrect dose, or toxic substances.7 The consequences are dire, leading to treatment failure, prolonged illness, the rise of antimicrobial resistance from inadequate dosages, and, in the worst cases, death.9 More than half of the counterfeit pharmaceuticals sold today are fraudulent versions of treatments for life-threatening conditions such as malaria, tuberculosis, HIV/AIDS, and cancer, directly targeting the world’s most vulnerable patients.6
A Global Threat
While often framed as a problem for the developing world, pharmaceutical counterfeiting is a truly global challenge. The Pharmaceutical Security Institute (PSI) reported that incidents of counterfeiting increased by 38% between 2016 and 2020.9 In 2020, North America ranked first among regions with the highest number of counterfeit medicine seizures, accounting for 32% of incidents, followed by the Asia Pacific region at 23%.9 The primary vector for this penetration into developed markets is the internet. An estimated 96% of the 35,000 active online pharmacies selling prescription medicines to U.S. consumers in 2019 did not comply with applicable legal and pharmacy standards.9 The WHO has estimated that as much as 50% of drugs sold online are fraudulent, creating a massive and largely unregulated entry point into even the most secure national supply chains.6
Economic Ripple Effects
The economic damage inflicted by counterfeit drugs extends far beyond the direct revenue losses for legitimate manufacturers. These losses hinder their ability to invest in future research and development, stifling innovation.1 Furthermore, the presence of counterfeit drugs drives up overall healthcare costs. The National Association of Boards of Pharmacy (NABP) estimates an annual loss to the global economy of $75 billion due to increased medical expenses and lost income from prolonged illnesses caused by ineffective treatments.1 This places an immense strain on healthcare systems, which must divert resources to treat complications arising from fake drugs, and it erodes the foundational public trust in medicines, doctors, and the healthcare system as a whole.1
1.2 The Labyrinthine Supply Chain: A System Riddled with Inefficiency
The modern pharmaceutical supply chain is a marvel of global logistics, yet it is also a system characterized by opacity, fragmentation, and staggering waste. These inefficiencies not only impact corporate bottom lines but also directly affect patient access to essential medicines.
The “Missing Billions”
A comprehensive study revealed that over 7.1% of all pharmaceutical stock is lost within the supply chain. This figure is composed of 4.1% of stock that perishes, spoils, or is damaged, and an additional 3% that is lost due to overproduction.2 This level of waste is estimated to be equivalent to a loss of 3.6% of annual profits for pharmaceutical firms, translating to an astonishing $10.3 billion in terms of the sale value of the lost stock.2 The primary drivers of this waste are issues with product packaging, spoilage of perishable items, and inefficient transport and delivery logistics.2
The Chronic Pain of Drug Shortages
Parallel to the problem of waste is the persistent and worsening crisis of drug shortages. According to data from the University of Utah Drug Information Service, the number of drugs in short supply in the U.S. reached a record high of 323 in the first quarter of 2024, surpassing the previous peak set in 2014.3 These shortages disproportionately affect generic drugs, which account for 90% of all prescriptions, and critical injectable products, which made up half of all drug shortages between 2018 and 2023.3
The causes are multifaceted and systemic. They include quality control issues at manufacturing facilities, which can lead to production halts; razor-thin profit margins on older generic drugs, which disincentivize production; and an increasingly globalized and fragile supply chain.3 As of 2024, only 24% of active pharmaceutical ingredient (API) manufacturing facilities for U.S.-marketed drugs were located within the United States, creating significant dependencies on international partners and exposing the supply chain to geopolitical disruptions.10 The consequences for patients are severe, leading to delays in medical care, higher rates of medication errors as alternatives are substituted, and, in some documented cases, elevated mortality rates.10
Opacity and Fragmentation
At the heart of these inefficiencies lies a fundamental lack of transparency. The pharmaceutical supply chain typically operates on a “one-up, one-down” visibility model, where each participant can only see the transaction immediately preceding and following their own.12 This creates deep information silos, making it impossible for stakeholders to have a holistic, end-to-end view of the supply chain.13 This opacity prevents effective inventory management, hinders the ability to respond to sudden demand surges—as was brutally exposed during the COVID-19 pandemic—and makes the process of recalling a specific batch of drugs slow, manual, and overly broad.14
1.3 The Crisis of Credibility: Data Integrity in Clinical Research
The development and approval of new medicines rely on the absolute integrity of clinical trial data. Any compromise in the accuracy, reliability, or completeness of this data can have consequences that are even more severe than manufacturing or supply chain failures, as it can lead to the approval of unsafe drugs or the rejection of effective ones.
The High Stakes of Data
Good Clinical Practice (GCP) data integrity issues can be crippling for a pharmaceutical company. In the most severe cases, the U.S. Food and Drug Administration (FDA) can completely reject the data submitted in a new drug application, rendering years of research and hundreds of millions of dollars in investment worthless.4 This was exemplified in a high-profile 2015 case where an FDA inspection of a contract research organization (CRO) in India found evidence of subject plasma sample manipulation, leading to the need for sponsors to repeat costly bioequivalence studies.4
A Pervasive Problem
Data integrity issues are not rare occurrences. An FDA review of 208 Clinical Inspection Summaries from 2015 and 2016 found that 37 of them (18%) contained “important” GCP-related recommendations specifically intended to address data integrity concerns.4 More strikingly, in a recent six-month pilot program launched as part of the Generic Drug User Fee Amendments (GDUFA III), the FDA found that data integrity issues were the primary reason for 75% of the delays in approving Abbreviated New Drug Applications (ANDAs) past their target goal date.5
The Root Causes
The most common inspection findings that lead to these issues are systemic rather than isolated. They include a failure by clinical investigators and sponsors to follow the investigational plan, the maintenance of inadequate and inaccurate records, and inadequate monitoring of trial sites by sponsors.4 These findings point to fundamental weaknesses in the traditional processes of data capture, verification, and management, which often rely on a complex and manual chain of custody as data moves from the patient to the sponsor and ultimately to the regulator.
The challenges of counterfeiting, supply chain inefficiency, and data integrity are not three separate problems. They are, in fact, deeply intertwined manifestations of a single, foundational flaw in the pharmaceutical industry’s architecture: the lack of a shared, immutable, and verifiable source of truth. Counterfeit drugs infiltrate the supply chain because there is no single, trusted ledger that can definitively prove a drug’s provenance from creation to dispensation.15 Supply chain inefficiencies are magnified by the existence of countless separate, siloed ledgers, which prevent a real-time, holistic view of inventory and demand.14 Clinical trial data remains vulnerable because it is typically stored in centralized, alterable databases, with its integrity dependent on a chain of human trust rather than cryptographic certainty.4 Any solution that addresses only one of these symptoms—a new hologram on a package, a better forecasting software, a more stringent auditing protocol—is merely a temporary patch. The industry requires a new foundational layer, a technological architecture that can establish a single, decentralized source of truth that no single competitor controls and that cannot be tampered with. This is the precise challenge that blockchain technology was designed to solve.
Part II: Blockchain as a Foundational Layer of Trust
To address the systemic vulnerabilities outlined in Part I, the pharmaceutical industry requires more than incremental improvements; it needs a new technological foundation capable of establishing trust in a decentralized and fragmented ecosystem. Blockchain, or Distributed Ledger Technology (DLT), provides this foundation. This section will demystify the core principles of blockchain from a strategic business perspective and map these principles directly to the industry’s most pressing challenges.
2.1 Beyond the Hype: A C-Suite Guide to Blockchain Principles
At its core, blockchain is an advanced database mechanism that enables transparent and secure information sharing within a business network.19 While often associated with cryptocurrencies, its fundamental features have far broader applications, particularly in industries where trust, security, and traceability are paramount.
Decentralized Ledger Technology (DLT)
Unlike a traditional database, which is typically centralized and controlled by a single entity (like a bank or a corporation), a blockchain is a decentralized network.20 The ledger—a record of all transactions—is not stored in one place but is copied and distributed across multiple computers, known as nodes, in the network.21 This decentralization is a critical feature for an industry of competitors. It means that no single participant—be it a manufacturer, wholesaler, or regulator—owns or controls the data.19 Instead, control is distributed among all permissioned members of the network, eliminating the single point of failure and control that characterizes centralized systems and fostering a “trustless” environment where participants do not need to inherently trust each other to trust the data on the ledger.20
Immutability and Cryptography
The name “blockchain” comes from how data is structured. Transactions are recorded in “blocks,” and once a block is full, it is cryptographically linked to the previous block, forming a chronological “chain”.19 Each block contains a unique cryptographic hash of the previous block’s data. If any information in a past block is altered, its hash will change, which would in turn change the hash of every subsequent block, effectively breaking the chain.24 This cryptographic linkage makes the ledger immutable—meaning the data entered is irreversible and cannot be altered or deleted without being detected by the entire network.20 This creates a permanent, tamper-evident, and verifiable audit trail for every transaction ever recorded.25
Consensus and Transparency
Before a new block of transactions can be added to the chain, it must be validated by the network participants. This is achieved through a “consensus mechanism,” a set of pre-agreed rules that govern the network.19 In a permissioned blockchain, which is most common for business applications, a new transaction is only recorded when the majority of designated participants agree that it is valid.20 Once consensus is reached, the new block is added to the chain, and the updated ledger is distributed to all participants in real-time.19 This process ensures that all permissioned stakeholders have access to the same version of the information at all times, creating a shared, single source of truth and a high degree of transparency.20
Smart Contracts
A smart contract is a self-executing program stored on the blockchain that automatically runs when predetermined conditions are met.19 These contracts operate on simple “if-then” logic. For example, a smart contract could be programmed with the rule: “IF the IoT sensor on the shipment confirms the product has arrived at the pharmacy’s location AND the product’s authenticity is verified on the blockchain, THEN automatically release payment from the pharmacy’s account to the wholesaler’s account.” By automating these steps, smart contracts can dramatically reduce administrative costs, eliminate the need for intermediaries, speed up settlements, and reduce the potential for human error or disputes.26
2.2 Mapping Industry Challenges to Blockchain Solutions
The true strategic value of blockchain lies in its ability to directly address the root causes of the pharmaceutical industry’s core challenges. By applying the principles described above, a clear problem-solution framework emerges.
- To Combat Counterfeits: The fundamental problem enabling counterfeit drugs is the lack of a verifiable, end-to-end record of provenance. Blockchain’s decentralized and immutable ledger provides exactly this. By creating a digital “pedigree” for each drug package that is tracked and verified at every step of the supply chain, blockchain makes it possible for any stakeholder, from a customs agent to a pharmacist, to instantly verify a drug’s authenticity against a trusted, shared record.15
- To Overcome Inefficiencies: The root cause of supply chain waste and shortages is the fragmentation of data across siloed systems. Blockchain’s shared, real-time ledger breaks down these silos, providing all permissioned participants with unprecedented visibility into inventory levels, shipment locations, and demand signals across the entire network.25 This shared visibility allows for better forecasting, more efficient logistics, and rapid, targeted responses to disruptions. Furthermore, smart contracts can automate routine processes like order fulfillment, invoicing, and payments, significantly reducing administrative overhead and accelerating the cash-to-cash cycle.29
- To Ensure Data Integrity: The vulnerability of clinical trial data stems from its storage in centralized, alterable databases. Blockchain provides a tamper-proof and time-stamped ledger for every piece of clinical data. Once a result is recorded on the chain, it cannot be secretly modified or deleted.15 This creates an unimpeachable audit trail that guarantees the integrity of the research data for sponsors, monitors, and regulatory agencies, fostering greater trust in the outcomes of clinical trials.25
The following table provides a concise summary of this mapping, illustrating how blockchain’s core features provide a direct architectural solution to the industry’s most significant operational and trust-related challenges.
| Industry Challenge | Systemic Root Cause | Enabling Blockchain Principle | Business Outcome |
| Counterfeit Drugs | Opaque Provenance; Lack of a single, verifiable product history. | Decentralized, Immutable Ledger for End-to-End Traceability | Guaranteed product authenticity; Enhanced patient safety; Brand protection. |
| Supply Chain Inefficiency | Information Silos; Lack of real-time, end-to-end visibility; Manual, paper-based processes. | Shared Real-Time Data Ledger; Smart Contract Automation | Enhanced visibility; Reduced waste and shortages; Faster, more accurate recalls; Lower administrative costs. |
| Clinical Data Tampering | Centralized, alterable databases; Lack of a verifiable, time-stamped audit trail. | Cryptographic Security & Consensus; Immutable Ledger | Verifiable data integrity for regulators; Increased trust in research outcomes; Secure patient consent management. |
| Intellectual Property Theft | Lack of verifiable, time-stamped proof of invention and R&D milestones. | Time-stamped & Immutable Records | Defensible proof of invention; Secure management of trade secrets; Streamlined IP licensing. |
This direct alignment between problem and solution demonstrates that blockchain is not a technology in search of a problem. Instead, it represents a fundamental architectural shift from a collection of disparate systems-of-record, each controlled by a single entity, to a unified system-of-trust, where the validity of the record is guaranteed by the collective, decentralized network. In the supply chain, trust is moved from paper pedigrees to a shared, immutable ledger. In clinical trials, trust is externalized from human auditors to the cryptographic certainty of the chain. This conceptual shift is the true revolution, re-architecting how trust is established and maintained in a multi-trillion-dollar industry.
Part III: Real-World Applications: From Theory to Tangible Value
The principles of blockchain technology translate into a wide array of practical applications across the pharmaceutical value chain. These applications move beyond theoretical benefits to create tangible value by enhancing security, efficiency, and transparency. This section explores the primary use cases in detail, supported by evidence from pilot programs and early-stage implementations.
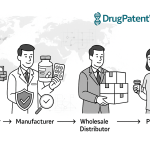
3.1 Securing the Global Supply Chain: The Quest for End-to-End Traceability
The most mature and widely explored application of blockchain in the pharmaceutical industry is in securing the supply chain. Its ability to create a transparent and immutable record of a product’s journey is a natural fit for an industry grappling with counterfeiting and regulatory compliance.
Achieving DSCSA Compliance and Beyond
In the United States, the Drug Supply Chain Security Act (DSCSA) mandates the creation of an electronic, interoperable system to identify and trace prescription drugs down to the package level by 2023.30 Blockchain has emerged as a leading technology to meet these requirements. Pilot programs have successfully demonstrated that a blockchain-based network can connect the disparate systems of manufacturers, distributors, and dispensers to create a common, shared record of product movement, fulfilling the core interoperability mandate of the DSCSA.31 This shared infrastructure reduces the individual burden on each company to build custom point-to-point connections, creating a more efficient and scalable industry-wide solution.32
Real-time Provenance and Counterfeit Detection
The operational model for a blockchain-enabled supply chain is straightforward yet powerful. Each saleable unit or batch of a drug is assigned a unique digital identifier, such as a serial number embedded in a QR code or 2D barcode.28 This identifier is recorded on the blockchain by the manufacturer, creating the first “block” in that product’s digital life story. As the product moves through the supply chain—from the manufacturing plant to a wholesaler’s distribution center, to a pharmacy, and finally to the point of dispensation—it is scanned at each handover point. Each scan creates a new transaction that is validated by the network and added as a new, time-stamped, and geographically-located block to the chain.15
This process creates an unbroken and immutable chain of custody. At any point, an authorized stakeholder can scan the product’s code and instantly access its entire provenance on the blockchain, verifying that it originated from a legitimate manufacturer and followed an authorized distribution path.28 This makes it exceedingly difficult for counterfeit products, which lack a valid digital pedigree on the blockchain, to be introduced into the legitimate supply chain. If a counterfeiter attempts to copy a legitimate barcode, the ledger would flag the suspicious activity, as a single unique identifier cannot be in two places at once or be dispensed multiple times.15
Innovating the Cold Chain
The integration of Internet of Things (IoT) sensors with blockchain technology offers a revolutionary solution for managing the cold chain—the temperature-controlled supply chain required for sensitive biologics, vaccines, and other specialty drugs. IoT sensors placed within shipping containers can continuously monitor critical environmental conditions like temperature and humidity.28 This real-time data can be automatically and directly recorded onto the blockchain, creating a verifiable and tamper-proof log of the product’s environmental history.28
The power of this integration is amplified by smart contracts. A smart contract can be programmed with specific rules, such as: “The temperature for this vaccine shipment must remain between 2°C and 8°C.” If an IoT sensor detects a temperature excursion outside this range and records it to the blockchain, the smart contract can automatically trigger a series of actions. It could send an immediate alert to the manufacturer and logistics provider, change the status of the shipment to “non-saleable” to prevent it from being administered to a patient, or even halt the payment process for the compromised shipment.28 This automated monitoring and enforcement system ensures product efficacy, enhances patient safety, and reduces waste from spoiled products.
3.2 Reinventing Clinical Trials: Forging an Immutable Chain of Evidence
The clinical trial process, the bedrock of pharmaceutical R&D, is another area ripe for blockchain-driven transformation. By ensuring the integrity of data and streamlining complex processes, blockchain can increase the credibility and efficiency of clinical research.
Ensuring Verifiable Data Integrity
Blockchain provides a secure and tamper-proof ledger for all clinical trial data. Every piece of information—from patient-reported outcomes and data from wearable devices to lab results and case report forms—can be cryptographically hashed, time-stamped, and recorded on the chain.18 Once a record is added, it cannot be altered or deleted without leaving a detectable trace. This immutability ensures the integrity of the clinical trial data, providing a verifiable and auditable trail for monitors, sponsors, and regulatory bodies.15 This helps to prevent scientific misconduct such as data dredging (selectively analyzing data to find a significant result) or p-hacking (manipulating data to achieve statistical significance), thereby increasing trust in the research findings.18 A Japanese breast cancer clinical trial successfully incorporated a blockchain-based system to demonstrate its resilience to data tampering, even during a major cloud server shutdown.18
Automating Patient Consent and Control
The management of informed consent is a critical and often cumbersome aspect of clinical trials. Blockchain, through the use of smart contracts, can automate and secure this process. A patient’s consent can be recorded as a transaction on the blockchain, creating an immutable and time-stamped record.27 Patients can be given control over their data via a private key, allowing them to grant, revoke, or set specific, time-limited permissions for how their data is used and who can access it.18 This not only enhances patient privacy and agency but also provides sponsors with a clear, auditable trail of consent that is crucial for compliance with regulations like GDPR and HIPAA. This patient-centric model may also ease the screening and recruitment process by allowing researchers to identify potential participants based on their disease profiles, with the patient’s explicit and verifiable permission.18
Facilitating Secure Multi-Stakeholder Collaboration
Drug discovery and development is an increasingly collaborative endeavor. Blockchain platforms can create a secure and neutral ground for pharmaceutical companies, academic research institutions, and CROs to share research data without relying on a central intermediary.27 By providing a shared, trusted ledger, blockchain can foster collaboration, accelerate the pace of discovery, and allow for more efficient development of new treatments while ensuring that intellectual property contributions are accurately tracked and recorded.27
3.3 The New Frontier: Patient-Centric Data and Intellectual Property
Beyond the supply chain and clinical trials, blockchain is poised to transform two other critical areas: the management of patient health records and the protection of intellectual property.
Empowering Patients with Control over Electronic Health Records (EHRs)
One of the most significant challenges in modern healthcare is the fragmentation of patient data across siloed EHR systems in different hospitals and clinics. Blockchain offers a potential solution by creating a single, comprehensive, patient-centric view of a medical record.21 In such a system, the actual sensitive health data is not stored directly on the blockchain. Instead, each new medical record (e.g., a lab result, a doctor’s note) is encrypted and stored off-chain (e.g., in a secure cloud database). A reference to this data, in the form of a cryptographic hash, is then recorded on the blockchain along with access permissions.37
The patient holds the private cryptographic key that controls access to their records. When visiting a new doctor, the patient can use their key to grant that provider temporary, view-only access to their complete medical history on the blockchain.37 This model breaks down data silos and ensures care continuity while placing the patient in ultimate control of their own health information, a paradigm shift from the current provider-centric model.38
Creating an Indelible Timestamp for Intellectual Property
In the hyper-competitive world of pharmaceutical R&D, establishing the precise timing of an invention can be critical for securing patent rights. Blockchain can serve as a digital notary, creating a secure, time-stamped, and immutable record of R&D activities.27 Researchers can hash their lab notes, discovery data, and invention disclosures and record these hashes on a blockchain. This provides irrefutable, verifiable proof that a specific piece of information existed at a specific point in time.39 While not a substitute for filing a patent, this immutable record can serve as powerful evidence of creatorship and the date of invention, which can be invaluable in patent disputes or for protecting valuable trade secrets that are not patented.27
Part IV: The Vanguard in Action: In-Depth Case Studies of Industry Pioneers
The theoretical applications of blockchain are being put to the test in the real world by a vanguard of pharmaceutical companies, technology providers, and regulatory bodies. These initiatives, ranging from industry-wide consortia to targeted enterprise solutions, provide invaluable insights into the practical implementation, challenges, and tangible benefits of blockchain technology. This section provides an in-depth analysis of these pioneering efforts.
4.1 The Consortium Model: Building the “Team Sport” Infrastructure
Recognizing that the value of a shared ledger is maximized when the network is broad and inclusive, many of the most significant blockchain initiatives have taken the form of industry consortia. As executives at Novartis have noted, “blockchain is a team sport,” requiring collaboration even among competitors to build the necessary infrastructure and standards.41
MediLedger (Chronicled)
The MediLedger Network stands as one of the most prominent and mature examples of an industry-led blockchain consortium in the U.S.
- Genesis & Goals: The project was born out of a direct business need: to solve the saleable returns verification requirement of the DSCSA. It was initiated by a core group of industry giants, including manufacturers Genentech (a member of the Roche Group) and Pfizer, and two of the “big three” wholesalers, McKesson and AmerisourceBergen.31 The challenge was to create a system where a wholesaler could verify the authenticity of a returned drug package with the original manufacturer without revealing sensitive business data to competitors on the network.
- Evolution: MediLedger successfully demonstrated a “start with compliance, scale to value” strategy. After proving its utility for DSCSA returns, the network expanded its scope to address more complex and high-value commercial pain points. Its current solutions now focus on aligning contract pricing and customer eligibility between manufacturers, wholesalers, Group Purchasing Organizations (GPOs), and health systems to eliminate costly chargeback errors and disputes.43 This evolution shows how an initial regulatory driver can become a foundation for broader business process optimization.
- Technology & Governance: The network is built on a permissioned blockchain (originally a version of Ethereum, with a stated relationship with Parity, the developer behind the interoperability-focused Polkadot network) that uses zero-knowledge proofs to ensure data privacy.42 A cornerstone of the MediLedger philosophy is an industry-owned and governed model, designed to prevent any single entity or industry segment from having undue influence over the network’s rules and direction.42 The success of this approach is validated by testimonials from key participants. Ray Pelliccioti, Director of Enterprise Operations at Johnson & Johnson, noted that the blockchain provides “real-time updates to roster and contract communications, reducing errors,” while Bill Marquardt, Vice President at the GPO Premier Inc., stated the approach “has the potential to transform how chargebacks work for the industry”.43
PharmaLedger (IMI)
Representing the European approach, PharmaLedger is a massive public-private partnership funded by the Innovative Medicines Initiative (IMI), a joint undertaking of the European Union and the European Federation of Pharmaceutical Industries and Associations (EFPIA).45
- Scope: This ambitious 36-month project, which concluded in December 2022, brought together a consortium of 29 partners, including 12 global pharmaceutical companies like Novartis, Pfizer, Johnson & Johnson, Bayer, and AstraZeneca, alongside 17 public and private entities from academia, technology, and patient advocacy groups.46
- Broad Mandate: Unlike the initial narrow focus of U.S. initiatives, PharmaLedger was designed from the outset to create a common, foundational blockchain framework for the entire European healthcare ecosystem. Its use cases were broad, covering the supply chain (e.g., finished goods traceability, anti-counterfeiting), clinical trials (e.g., eConsent, clinical supply chain), and health data (e.g., managing medical information).46
- Patient-Centric Focus: A distinguishing feature of PharmaLedger is its strong emphasis on patient-centricity. The European Patients’ Forum (EPF) was an integral partner, contributing to the project’s governance and the design of a “Collaboration Platform” to ensure the patient perspective was embedded in the technology’s development. The project’s ultimate goal was to accelerate innovation that benefits patients, improves the security of their health data, and speeds the delivery of medicines.46
4.2 The Regulatory Sandbox: The FDA DSCSA Pilot Project
A landmark pilot program, announced by the FDA in 2019, brought together four corporate giants—IBM, KPMG, Merck, and Walmart—to formally test blockchain’s viability for meeting the 2023 DSCSA interoperability requirements.32
- Participants & Objectives: The collaboration was designed to demonstrate that a blockchain network could connect the disparate existing systems of a manufacturer (Merck), a distributor/dispenser (Walmart), and technology/consulting providers (IBM, KPMG) to create a common record of product movement and enhance patient safety during recalls.32
- Key Findings: The pilot, whose final report was published in February 2020, was a resounding success. It proved that drug provenance could be accurately captured on the blockchain while maintaining data privacy through a permissioned “one up, one down” view.32 Most impressively, the pilot demonstrated a dramatic improvement in patient safety capabilities. The time required to identify affected products and alert downstream partners during a recall was reduced from a manual process that can take up to three days to as little as ten seconds using the blockchain solution.12
- Strategic Conclusion: The participants collectively concluded that blockchain not only offers a trusted, decentralized path to DSCSA compliance but also unlocks significant value beyond regulation. They identified potential future applications in managing cold chain logistics, addressing drug shortages, and optimizing inventory.32 The public statements from executives involved underscored the project’s impact. Craig Kennedy, Senior Vice President of Supply Chain at Merck, stated, “Reliable and verifiable supply helps improve confidence among all the stakeholders—especially patients”.47 Mark Treshock, IBM’s Global Solutions Leader for Blockchain in Healthcare, added, “Blockchain has the potential to transform how pharmaceutical data is controlled, managed, shared and acted upon throughout the lifetime history of a drug”.47
4.3 Enterprise Solutions in Focus: A Comparative Analysis
Alongside consortia, several major technology and specialized startup companies are developing enterprise-grade blockchain solutions for the pharmaceutical industry.
- SAP: The enterprise software giant has developed the Information Collaboration Hub for Life Sciences, a blockchain solution focused on product verification and DSCSA compliance. Through co-innovation partnerships with pharmaceutical companies like Boehringer Ingelheim, SAP’s system allows manufacturers to record unique product identifiers on the blockchain. This enables anyone in the supply chain, including the end consumer with a smartphone, to scan a package’s 2D barcode and instantly verify its serial number and authenticity against the immutable ledger.33
- FarmaTrust: This specialized platform leverages a combination of blockchain and Artificial Intelligence (AI) to secure the pharmaceutical supply chain.24 Their Zoi system is designed to be interoperable with existing enterprise software, providing an end-to-end tracking solution that creates an unbroken chain of custody to prevent counterfeit drugs from entering the market.16 A key real-world application of their technology was a pilot project conducted in partnership with the Mongolian government. The project aimed to track and trace the national pharmaceutical supply chain to combat the prevalence of fake medicines, demonstrating the scalability of the solution from a corporate to a national level.49
- Guardtime: A cybersecurity firm with deep roots in blockchain technology, Guardtime utilizes its proprietary Keyless Signature Infrastructure (KSI) blockchain as a “trust anchor” to guarantee data integrity. In the healthcare space, they have notably partnered with pharmaceutical leaders Roche and AstraZeneca to develop a real-world data engine.52 This innovative solution allows for the analysis of aggregated medicine usage data from health registries across a population without revealing any personal health information. This enables advanced applications like value-based pricing, indication-based pricing, and more efficient patient enrollment for clinical trials, all while preserving patient privacy.52
- Other Pioneers: The landscape is rich with other innovators targeting specific niches. BlockRx, an initiative by iSolve, aims to create a comprehensive platform using its Advanced Digital Ledger Technology™ (ADLT™) to fully integrate life science researchers, biopharma companies, and healthcare providers to improve patient outcomes.54
Spiritus Partners has worked on projects, including a collaboration with Edinburgh Napier University and the UK’s National Health Service (NHS), to investigate using blockchain to track the lifecycle of medical devices across complex ecosystems.55
Medicalchain is focused on the patient-centric data model, using blockchain to securely store health records and empower patients with control over their data through their platform and the MyClinic.com telemedicine application.57
The following table provides a comparative overview of these major initiatives, offering a strategic snapshot of the key players, their focus areas, and their progress in deploying blockchain technology in the pharmaceutical sector.
| Initiative/Platform | Key Participants | Primary Focus Area | Key Finding / Status |
| MediLedger | Chronicled, Pfizer, McKesson, AmerisourceBergen, J&J, Premier Inc. | DSCSA Compliance, Contract & Chargeback Alignment | Operational network; expanded from returns verification to complex commercial contract alignment. |
| PharmaLedger | IMI, Novartis, Pfizer, J&J, Bayer, AstraZeneca, EPF, and 22 others | Pan-European Healthcare Framework (Supply Chain, Clinical Trials, Health Data) | Completed in 2022; established a foundational blockchain platform and governance model for Europe. |
| FDA DSCSA Pilot | IBM, KPMG, Merck, Walmart | Drug Traceability, Recall Management, DSCSA Interoperability | Successful pilot; reduced recall alert times from up to 3 days to as little as 10 seconds. |
| SAP Pharma Blockchain | SAP, Boehringer Ingelheim | Product Authenticity, DSCSA Compliance | Enables consumer-level verification of drug authenticity via barcode scanning. |
| FarmaTrust | FarmaTrust, Mongolian Government | Anti-Counterfeiting, Supply Chain Integrity (Blockchain + AI) | Successfully piloted a national-level drug tracking system in Mongolia. |
| Guardtime | Guardtime, Roche, AstraZeneca | Real-World Data Analysis, Value-Based Pricing | Developed a privacy-preserving engine for analyzing aggregated medicine usage data. |
Part V: Strategic Imperative: Turning Patent Data into Competitive Advantage
In the high-stakes pharmaceutical industry, intellectual property (IP) is not just a legal asset; it is a critical driver of value and a primary source of competitive intelligence. As blockchain technology matures from a conceptual novelty into a strategic enabler, the landscape of related patents is becoming a crucial battlefield. For discerning companies, analyzing this landscape provides a powerful tool to forecast market trends, identify threats, and uncover opportunities for innovation. This section provides a strategic framework for leveraging patent data, with a practical guide on using specialized platforms like DrugPatentWatch to gain a competitive edge.
5.1 The Patent Landscape as a Strategic Battlefield
Patents are a rich source of information that, when properly analyzed, can reveal a wealth of intelligence about competitors’ activities, R&D priorities, emerging technological fields, and potential collaborations.59 In the context of a transformative technology like blockchain, monitoring the patent landscape is essential for any company seeking to innovate and protect its market position.
The Blockchain Patent Surge
The strategic importance of blockchain is reflected in the rapid growth of patent filings related to the technology. The global market for blockchain patents was valued at $228 million in 2020 and is projected to reach $703 million by 2025, demonstrating a compound annual growth rate of 25.3%.60 This “rush to patent” by both technology firms and incumbent industry players signals a clear belief in the long-term value of blockchain-related IP.61 For pharmaceutical companies, this means that failing to monitor this rapidly evolving landscape is a significant strategic risk. It could lead to being blocked by a competitor’s broad patent, missing out on a key licensing opportunity, or investing in R&D that infringes on existing IP.
Beyond Simple Tracking: The Importance of Claims Analysis
Effective competitive intelligence from patents requires moving beyond simply counting the number of patents filed by a competitor. The true value lies in a deep analysis of the patent claims. The claims are the legally operative part of a patent, defining the precise boundaries of the invention that is protected.62
Analyzing the claims allows a company to understand a competitor’s strategic intent. For example:
- Broad vs. Narrow Claims: A patent with very broad claims may indicate an attempt to dominate a foundational area of the technology, but it may also be more vulnerable to legal challenges. Narrow, specific claims might protect a particular application or improvement, revealing a more focused product strategy.62
- Method vs. System Claims: A “method” claim protects a specific process (e.g., “a method for verifying drug authenticity using a distributed ledger”). A “system” claim protects a technological architecture (e.g., “a system comprising IoT sensors and a blockchain for cold chain management”). Understanding this distinction reveals whether a competitor is focused on protecting a business process or a core piece of technology.64
By dissecting the claims of patents filed by rivals, a company can build a detailed picture of their technological roadmap, their areas of focus, and their potential future products.
5.2 A Practical Guide: Leveraging DrugPatentWatch for Blockchain Intelligence
Specialized business intelligence platforms are essential for navigating the complex world of pharmaceutical patents. DrugPatentWatch is a prime example of a platform that focuses on drug patent dynamics and provides the tools necessary to transform raw patent data into actionable competitive intelligence.65 The following is a practical, step-by-step guide for using such a platform to analyze the blockchain patent landscape.
Step 1: Defining the Search Strategy
A successful analysis begins with a well-crafted search. The goal is to cast a wide net initially and then systematically refine the results.62
- Brainstorming Keywords: The search should combine blockchain-related terms with pharma-specific applications. Using Boolean operators is crucial for precision. For example, a search query might look like: (“blockchain” OR “distributed ledger” OR “smart contract”) AND (“pharmaceutical supply chain” OR “clinical trial” OR “drug provenance” OR “electronic health record”). This ensures the results are relevant to the pharmaceutical industry.62
- Identifying Assignees: A key strategy is to track the patent filings of specific organizations. This includes the technology pioneers identified in Part IV (e.g., IBM, SAP, Chronicled, Guardtime) as well as the major pharmaceutical companies that are active in the space (e.g., Pfizer, Novartis, Merck, Johnson & Johnson). Searching by assignee allows for a direct view into the IP strategy of key competitors and partners.62
Step 2: Analyzing the Results – From Data to Intelligence
Once a relevant set of patents has been collected, the analysis phase begins. This is where raw data is transformed into strategic insight.
- Patent Landscaping: The first level of analysis involves creating a “patent landscape.” This is a visual representation of the patent data that can reveal macro trends.62 Tools within platforms like DrugPatentWatch can help answer key questions: Which companies are filing the most patents in this space? In which jurisdictions (e.g., U.S., Europe, China) is activity concentrated? Is the rate of patent filings accelerating, indicating a heating up of the technological race? This provides a high-level map of the competitive terrain.60
- Deep Dive into Claims Analysis: This is the most critical and resource-intensive step. It requires a careful reading and interpretation of the claims of the most relevant patents identified in the landscaping phase.62 The goal is to understand precisely what is being protected. For example, is a competitor like IBM patenting a novel consensus mechanism specifically designed for high-throughput supply chain transactions? This would indicate a deep, foundational technology play. Or is a pharmaceutical company patenting a specific method of using smart contracts to automate patient consent for clinical trials using data from their EHR? This would signal a more focused, application-level strategy.64
- Identifying Risks and Opportunities: The claims analysis directly informs risk assessment and opportunity identification. A “high-risk” patent is one with broad claims that could potentially cover a product or process your company is developing, creating a freedom-to-operate (FTO) issue.64 Conversely, the analysis can reveal “white spaces”—areas of technological or application focus with little to no patent activity. These white spaces represent potential opportunities for your company to innovate and establish a strong IP position.62 The analysis can also identify patents that are nearing their expiration date, which may open up new possibilities for building upon previously protected technology.62
Step 3: Integrating with Business Strategy
The intelligence gathered from the patent analysis must be integrated into the company’s broader strategic planning process.
- Informing R&D: The patent landscape should directly guide R&D efforts. It can help teams avoid crowded areas where the risk of infringement is high and steer them toward promising white spaces where they can become leaders.64
- Guiding M&A and Partnerships: The analysis can identify startups or other companies with valuable and innovative IP portfolios, making them attractive targets for mergers, acquisitions, or strategic licensing agreements. This allows a company to acquire technology and talent rather than building it from scratch.62
- Forecasting Market Shifts: The long-term trends in patent filings are a powerful leading indicator of future market dynamics. For example, a surge in patent filings that combine blockchain with genomics and personalized medicine would be a strong signal that this is a major emerging frontier for the industry, allowing your company to position itself accordingly.65
The following table provides a structured framework for conducting this type of analysis, transforming the concept of competitive intelligence into a repeatable and actionable business process.
| Phase | Action | DrugPatentWatch Application | Key Data to Extract | Strategic Question Answered |
| I. Scoping & Strategy | Define Key Technology Areas (e.g., Supply Chain, Clinical Trials, IP Management). | Use platform’s search filters and categories to align with strategic priorities. | Relevant patent classifications (e.g., IPC, CPC); list of key competitors and technology providers. | What specific problems are our competitors trying to solve with blockchain? |
| II. Data Collection & Filtering | Conduct Keyword & Assignee Searches. | Use advanced search with Boolean operators; filter results by assignee (e.g., ‘IBM’, ‘Pfizer’, ‘Novartis’). | Comprehensive list of patents filed by key competitors; filing dates and jurisdictions. | Who are the most active players in this space and where are they protecting their IP? |
| III. In-depth Analysis | Analyze Patent Claims for Scope and Focus. | Review the full text of individual patent documents, focusing on the “Claims” section. | Scope of claims (broad/narrow); type of claims (method/system); specific technologies mentioned. | What is the precise nature and strength of our competitors’ IP protection? |
| IV. Strategic Action | Identify “White Space” Opportunities and FTO Risks. | Use landscaping and visualization tools to map patent density; categorize patents by risk level. | Areas with low patent activity; patents with claims that overlap with internal R&D projects. | Where are the untapped innovation opportunities, and what are the potential IP roadblocks to our strategy? |
Part VI: The Path Forward: Navigating Hurdles and Embracing the Future
While the potential of blockchain technology in the pharmaceutical industry is immense, its widespread adoption is not a foregone conclusion. The path from promising pilot projects to industry-wide implementation is fraught with significant technological, organizational, and regulatory hurdles. A clear-eyed understanding of these challenges is essential for any organization seeking to navigate this transformation. Furthermore, the ultimate value of blockchain will likely be realized not in isolation, but through its convergence with other powerful technologies like Artificial Intelligence (AI) and the Internet of Things (IoT).
6.1 From Potential to Practice: Overcoming the Barriers to Adoption
The transition to a blockchain-enabled ecosystem requires more than just technological innovation; it demands a concerted effort to overcome deep-seated challenges.
Technological Hurdles
- Scalability: A primary technical concern is the ability of blockchain networks to handle the massive volume of transactions inherent in the global pharmaceutical supply chain. Public blockchains, like the one used for Bitcoin, have inherent limitations on transaction speed and throughput. While permissioned or private blockchains, which are better suited for enterprise use, offer significantly better performance, they still require careful architectural design to manage potentially billions of transactions per day without creating latency issues.69
- Interoperability: Perhaps the most significant technological barrier is the lack of interoperability between different blockchain solutions. If Merck is on an IBM-based blockchain and Pfizer is on a SAP-based one, and the two systems cannot communicate, the industry risks simply replacing old data silos with new, blockchain-based ones.70 The development and adoption of industry-wide standards for cross-chain communication, such as those being explored by organizations like the IEEE, are critical to creating a truly unified and interoperable ecosystem.72
- Integration with Legacy Systems: Pharmaceutical companies have invested billions of dollars in existing enterprise resource planning (ERP), manufacturing execution systems (MES), and supply chain management (SCM) software. Integrating a new blockchain layer with these complex legacy systems is a non-trivial technical challenge that requires significant resources and expertise.74
Organizational & Financial Hurdles
- Cost of Implementation: The upfront investment required to implement a blockchain solution is substantial. Costs include not only the technology platform itself but also the integration with legacy systems, the need for enhanced network infrastructure, and the high cost of hiring or training specialized talent.69 For many organizations, particularly smaller ones, projecting a clear return on this investment can be difficult, making it a significant financial risk.69
- The Skills Gap: There is a well-documented shortage of developers, architects, and project managers who possess deep expertise in blockchain technology. This scarcity of talent makes recruitment difficult and expensive, and it can be a major bottleneck for companies looking to build and deploy blockchain solutions.76
- Stakeholder Resistance and Governance: The most formidable challenges are often not technical but human. Blockchain requires a paradigm shift from a competitive, zero-sum mindset to a collaborative one. Convincing fierce competitors to join a shared network and agree on a common set of rules is a massive organizational hurdle.45 Establishing an equitable governance model for a consortium—one that defines rules for data ownership, cost-sharing, and decision-making—is often more complex and critical to success than the underlying technology itself.45
Regulatory & Legal Hurdles
- Compliance with Data Privacy Laws: Navigating the complex web of data privacy regulations, especially the Health Insurance Portability and Accountability Act (HIPAA) in the U.S. and the General Data Protection Regulation (GDPR) in Europe, is a major challenge.79 The immutable nature of blockchain—the fact that data cannot be deleted—can be in direct conflict with regulations like GDPR’s “right to be forgotten.” Solutions often involve storing sensitive personal data off-chain, but this adds complexity and requires careful legal and technical design.81
- Regulatory Uncertainty: The legal and regulatory framework for blockchain technology is still in its infancy and continues to evolve.71 This uncertainty can create hesitation among companies, who may be reluctant to make significant investments in a technology whose legal standing is not yet fully settled.80
6.2 The Next Frontier: Synergies with AI, IoT, and Personalized Medicine
The long-term, transformative impact of blockchain will be realized through its convergence with other powerful technologies. Blockchain’s core value proposition—providing a secure, immutable, and trusted source of data—makes it the ideal foundational layer upon which AI and IoT can operate with greater reliability and impact.
The Intelligent Supply Chain (Blockchain + IoT + AI)
The future of the pharmaceutical supply chain is not just a traceable one, but an intelligent and autonomous one. In this converged model:
- IoT sensors provide a continuous stream of real-world data about a product’s location, condition, and environment.34
- Blockchain acts as the secure and immutable ledger, ensuring that this IoT data is trustworthy and has not been tampered with.24
- AI and machine learning models then analyze this trusted, real-time data to provide advanced insights. AI can predict potential supply chain disruptions, optimize logistics routes in real-time, identify patterns that indicate potential theft or diversion, and automate complex decisions through smart contracts.82 For example, an AI model could analyze data from a fleet of shipments and automatically execute a smart contract to reroute a critical vaccine shipment to avoid an impending weather event, all based on trusted data from the blockchain.
AI-Powered Clinical Trials on a Blockchain Foundation
The synergy between AI and blockchain also holds immense promise for revolutionizing clinical trials.
- AI can dramatically accelerate and optimize the process by analyzing vast datasets (EHRs, genomic data, etc.) to identify the most suitable patient cohorts for a trial, thereby speeding up recruitment.84 AI can also help in optimizing trial protocol design and predicting patient responses to treatment.86
- Blockchain provides the essential trust layer. It ensures the integrity and security of the vast amounts of data being fed into these AI models. It also provides a secure and transparent mechanism for managing patient consent, ensuring that patients have control over how their data is used for AI-driven analysis.87
Genomics and Personalized Medicine
Perhaps the most forward-looking application lies in the field of genomics and personalized medicine. The ability to tailor treatments to an individual’s unique genetic makeup is the future of healthcare, but it relies on access to vast and sensitive genomic datasets.
- Blockchain offers a paradigm-shifting solution to this challenge. It can empower individuals to truly own and control their own genomic data.89 Through decentralized platforms and marketplaces, a patient could store their encrypted genomic data and use a smart contract to grant researchers temporary, anonymized access for specific studies in exchange for compensation (e.g., via a token system). This model could unlock a torrent of valuable data for research into personalized medicine while fundamentally respecting and protecting patient privacy and agency.90
The ultimate value proposition of blockchain is not as a standalone solution, but as the secure, foundational data integrity layer for the next generation of healthcare technologies. AI and machine learning are powerful analytical engines, but their outputs are only as reliable as the data they are trained on. IoT devices are prolific data generators, but that data is only valuable if it is trustworthy. Blockchain solves the “trusted data” problem. By guaranteeing that the data from supply chains, clinical trials, and patients is immutable and its provenance is verifiable, blockchain dramatically increases the value and reliability of AI-driven insights and IoT-enabled processes. The future is not a choice between these technologies; it is their convergence. Blockchain provides the trust, IoT provides the real-world data, and AI provides the intelligence. This synergistic relationship is the true source of long-term competitive advantage.
Conclusion: A Strategic Blueprint for the Blockchain-Enabled Pharmaceutical Enterprise
The pharmaceutical industry is at a crossroads. The systemic challenges of counterfeit drugs, supply chain fragility, and data integrity are no longer sustainable liabilities; they are urgent imperatives for fundamental change. This report has demonstrated that blockchain technology offers more than just an incremental improvement—it provides a new architectural foundation capable of addressing the industry’s core trust deficit. By transitioning from a fragmented landscape of isolated systems-of-record to a unified, collaborative system-of-trust, blockchain can create a pharmaceutical ecosystem that is more secure, transparent, efficient, and ultimately, more patient-centric.
The journey from theory to practice is already well underway. Pioneering consortia like MediLedger and PharmaLedger, landmark regulatory pilots with the FDA, and innovative enterprise solutions from companies like IBM, SAP, and FarmaTrust have moved blockchain from the realm of hype to the reality of tangible value. They have proven its ability to secure the supply chain, enhance data integrity in clinical trials, and empower patients with control over their health information. The analysis of the burgeoning blockchain patent landscape further underscores the technology’s strategic importance, revealing a new frontier for competitive differentiation and market leadership.
However, the path to widespread adoption is not without significant obstacles. Technological hurdles of scalability and interoperability, organizational challenges of cost and collaboration, and the complexities of an evolving regulatory environment must be navigated with strategic foresight. The ultimate vision for a blockchain-enabled pharmaceutical enterprise will be realized through the convergence of this foundational trust layer with the intelligence of AI and the real-world data of IoT, creating a truly responsive, predictive, and secure healthcare ecosystem.
Actionable Recommendations for Industry Leadership
The adoption of blockchain is not an IT project to be delegated; it is a strategic business transformation that demands executive vision, sponsorship, and a commitment to a new way of operating. For leaders poised to guide their organizations into this new era, the following actions provide a strategic blueprint:
- Educate and Evangelize: Leadership must champion the strategic value of blockchain beyond the IT department. The focus should be on solving core business problems—reducing fraud, improving efficiency, enhancing data integrity—rather than on the technology for its own sake. Building blockchain literacy at all levels of the organization is the first step toward fostering a culture that is receptive to this transformative change.
- Join the “Team Sport”: The greatest value of blockchain is realized at the network level. Organizations should not attempt to build proprietary, walled-off solutions. Instead, they should actively participate in or join established industry consortia. Engaging with initiatives like MediLedger or contributing to the development of industry standards is crucial for shaping the future of the ecosystem and ensuring interoperability.
- Start with Compliance, Scale to Value: The most successful adoption strategies begin with a focused, high-impact use case that addresses a clear and present need, often driven by regulation. Using a mandate like the DSCSA as a starting point to build a foundational blockchain capability allows an organization to gain experience, demonstrate ROI, and build momentum before scaling the solution to address broader, value-driven commercial opportunities like chargeback management or cold chain optimization.
- Invest in Patent Intelligence: The blockchain IP landscape is a dynamic and strategic battlefield. Organizations must establish a formal, continuous process for monitoring and analyzing blockchain-related patents. Leveraging specialized platforms like DrugPatentWatch to track competitors’ filings, identify technological white spaces, and assess freedom-to-operate risks is no longer optional; it is a critical component of any forward-looking R&D and business strategy.
- Build a Converged Technology Roadmap: The future is not about a single technology but about their synergistic integration. Strategic planning must look beyond blockchain as a standalone solution and develop a long-term roadmap that anticipates the convergence of blockchain with AI and IoT. This means building a data architecture and a set of capabilities that position the organization to leverage blockchain as the secure, trusted data foundation for future AI-driven analytics and IoT-enabled automation.
The transition to a blockchain-enabled pharmaceutical industry will be a marathon, not a sprint. It will require sustained investment, bold leadership, and an unprecedented level of collaboration. However, the companies that begin laying this foundation today—by building technological capabilities, fostering partnerships, and cultivating a strategic vision—will not only solve the enduring problems of the present but will also be the ones to define and dominate the future of trusted, transparent, and patient-centric healthcare.
Works cited
- The Economic Impact of Counterfeit Healthcare Products – TrueMed, accessed July 28, 2025, https://truemedinc.com/blog/the-economic-impact-of-counterfeit-healthcare-products/
- Over 7% of pharmaceutical drugs go to waste as supply chain managers bemoan damaged and excess inventory – PharmiWeb.com, accessed July 28, 2025, https://www.pharmiweb.com/press-release/2022-11-10/over-7-of-pharmaceutical-drugs-go-to-waste-as-supply-chain-managers-bemoan-damaged-and-excess-inventory
- Symptoms of Disruption in the Pharmaceutical Supply Chain – Art of Procurement, accessed July 28, 2025, https://artofprocurement.com/blog/supply-symptoms-of-disruption-in-the-pharmaceutical-supply-chain
- Communicating with FDA When Data Integrity Issues Arise During Clinical Trials, accessed July 28, 2025, https://www.fdli.org/2019/05/communicating-with-fda-when-data-integrity-issues-arise-during-clinical-trials/
- FDA official says data integrity issues are the main reason for ANDA delays | RAPS, accessed July 28, 2025, https://www.raps.org/news-and-articles/news-articles/2025/4/fda-official-says-data-integrity-issues-are-the-ma
- New defenses for an underestimated — and growing — menace Fighting counterfeit pharmaceuticals – PwC Strategy, accessed July 28, 2025, https://www.strategyand.pwc.com/gx/en/insights/2017/fighting-counterfeit-pharmaceuticals/fighting-counterfeit-pharmaceuticals.pdf
- Pharmaceutical Counterfeiting: Endangering Public Health, Society and the Economy | Fraser Institute, accessed July 28, 2025, https://www.fraserinstitute.org/sites/default/files/pharmaceutical-counterfeiting-endangering-public-health-society-and-the-economy.pdf
- Substandard and falsified medical products – World Health Organization (WHO), accessed July 28, 2025, https://www.who.int/news-room/fact-sheets/detail/substandard-and-falsified-medical-products
- Fighting the global counterfeit medicines challenge: A consumer-facing communication strategy in the US is an imperative, accessed July 28, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC9031510/
- Analysis of Drug Shortages, 2018-2023 – HHS ASPE, accessed July 28, 2025, https://aspe.hhs.gov/sites/default/files/documents/efa332939da2064fa2c132bb8e842bb5/Drug%20Shortages_Data%20Brief_Final_2025.01.10.pdf
- Causes and Consequences of Medical Product Supply Chain Failures – NCBI, accessed July 28, 2025, https://www.ncbi.nlm.nih.gov/books/NBK583734/
- Blockchain for Supply Chain – IBM Blockchain, accessed July 28, 2025, https://www.ibm.com/solutions/blockchain-supply-chain
- Blockchain technology in the pharmaceutical industry: a systematic review – PMC, accessed July 28, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC9137953/
- THE PANDEMIC AND THE SUPPLY CHAIN Addressing Gaps – Johns Hopkins Bloomberg School of Public Health, accessed July 28, 2025, https://publichealth.jhu.edu/sites/default/files/2023-04/pandemic-supply-chain.pdf
- How Pharmaceutical Industry is Leveraging Blockchain Technology – Rejolut, accessed July 28, 2025, https://rejolut.com/blog/blockchain-in-pharmacy/
- FARMATRUST – NET, accessed July 28, 2025, https://cisfunctionsstorage.blob.core.windows.net/cis-files/whitepaper/FTT_farmatrust.pdf
- Supply Chain Challenges in Pharmaceutical Manufacturing Companies: Using Qualitative System Dynamics Methodology – PMC, accessed July 28, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC6706717/
- Burgeoning blockchain pilots to prove value in clinical trials, accessed July 28, 2025, https://www.clinicaltrialsarena.com/news/blockchain-clinical-trials-2/
- What is Blockchain Technology? – AWS, accessed July 28, 2025, https://aws.amazon.com/what-is/blockchain/
- Blockchain Technology Explained: What is Blockchain and How Does It Work?, accessed July 28, 2025, https://freemanlaw.com/blockchain-technology-explained-what-is-blockchain-and-how-does-it-work-2/
- How Can a Blockchain Be Used in Business? – Impact Networking, accessed July 28, 2025, https://www.impactmybiz.com/blog/how-can-blockchain-be-used-in-business/
- Blockchain Facts: What Is It, How It Works, and How It Can Be Used – Investopedia, accessed July 28, 2025, https://www.investopedia.com/terms/b/blockchain.asp
- What is Blockchain for Business? – IBM, accessed July 28, 2025, https://www.ibm.com/think/topics/blockchain-for-business
- Role of Blockchain in Pharmaceutical Supply Chain – Debut Infotech, accessed July 28, 2025, https://www.debutinfotech.com/blog/blockchain-in-pharmaceutical-supply-chain-the-next-big-frontier
- The benefits of blockchain in pharma – BCS365, accessed July 28, 2025, https://bcs365.com/insights/the-benefits-of-blockchain-in-pharma
- Leveraging Blockchain Technology to Enhance Leadership Strategies in Financial Services, accessed July 28, 2025, https://www.selbyjennings.com/en-us/industry-insights/hiring-advice/leveraging-blockchain-technology-to-enhance-leadership-strategies-in-financial-services
- Blockchain in Pharmaceutical: Benefits, Uses, and Future – Webisoft Blog, accessed July 28, 2025, https://blog.webisoft.com/blockchain-in-pharmaceutical/
- Blockchain: A Game-Changer for Pharma Supply Chains – Binariks, accessed July 28, 2025, https://binariks.com/blog/blockchain-pharma-supply-chain/
- Blockchain Your Supply Chain: Advantages for Pharmaceutical and Logistics Executives, accessed July 28, 2025, https://www.cevalogistics.com/en/ceva-insights/blockchain-your-supply-chain
- Blockchain and The Drug Supply Chain Security Act (DSCSA) – MoFo Life Sciences, accessed July 28, 2025, https://lifesciences.mofo.com/topics/blockchain-and-the-drug-supply-chain-security-act
- Phase 2: Big Pharma expands its blockchain experiment | Supply …, accessed July 28, 2025, https://www.supplychaindive.com/news/big-pharma-blockchain-experiment-returns/525689/
- FDA DSCSA Blockchain Interoperability Pilot Project Report, accessed July 28, 2025, https://www.fda.gov/media/169883/download
- SAP debuts blockchain solution for counterfeit drugs | Supply Chain …, accessed July 28, 2025, https://www.supplychaindive.com/news/blockchain-curtail-counterfeit-medicine/546658/
- Blockchain and IoT in Pharmaceutical Supply Chains – IRMA-International.org, accessed July 28, 2025, https://www.irma-international.org/viewtitle/330030/?isxn=9781668457474
- Internet of Things (IoT)–blockchain-enabled pharmaceutical supply chain resilience in the post-pandemic era – HEP Journals, accessed July 28, 2025, https://journal.hep.com.cn/fem/EN/Y2023/V10/I1/82
- The 6 Roles of Blockchain Technology in Pharma’s Future – NASSCOM Community, accessed July 28, 2025, https://community.nasscom.in/communities/blockchain/6-roles-blockchain-technology-pharmas-future
- Blockchain Technology in Healthcare: Benefits and Case Studies | BGO Software, accessed July 28, 2025, https://www.bgosoftware.com/blog/blockchain-technology-in-healthcare-benefits-and-case-studies/
- 5 blockchain healthcare use cases in digital health – STL Partners, accessed July 28, 2025, https://stlpartners.com/articles/digital-health/5-blockchain-healthcare-use-cases/
- Blockchain and IP Law: A Match made in Crypto Heaven? – WIPO, accessed July 28, 2025, https://www.wipo.int/web/wipo-magazine/articles/blockchain-and-ip-law-a-match-made-in-crypto-heaven-40267
- Intellectual Property Technology – Questel, accessed July 28, 2025, https://www.questel.com/about-questel/intellectual-property-technology/
- Novartis explores blockchain’s potential for pharmaceuticals …, accessed July 28, 2025, https://www.ledgerinsights.com/novartis-pharma-blockchain/
- MediLedger: Pharmaceutical industry’s blockchain network – Ledger …, accessed July 28, 2025, https://www.ledgerinsights.com/mediledger-pharmaceutical-blockchain/
- Chronicled, accessed July 28, 2025, https://www.chronicled.com/
- MediLedger blockchain developer Chronicled raises $8.3m from True Global Ventures, accessed July 28, 2025, https://www.ledgerinsights.com/mediledger-blockchain-founder-chronicled-raises-8-3m-from-true-global-ventures/
- CA GOV OPS BLOCKCHAIN WORKING GROUP – California …, accessed July 28, 2025, https://www.govops.ca.gov/wp-content/uploads/sites/11/2020/04/Pharmaceuticals-Item-11.pdf
- PharmaLedger – European Patients’ Forum, accessed July 28, 2025, https://www.eu-patient.eu/projects/completed-projects/pharmaledger/
- IBM, KPMG, Merck and Walmart to collaborate as part of FDA’s program to evaluate the use of blockchain to protect pharmaceutical product integrity, accessed July 28, 2025, https://www.merck.com/news/ibm-kpmg-merck-and-walmart-to-collaborate-as-part-of-fdas-program-to-evaluate-the-use-of-blockchain-to-protect-pharmaceutical-product-integrity/
- Verifying Pharmaceutical Products Using Blockchain – SAP, accessed July 28, 2025, https://www.sap.com/assetdetail/2018/05/c48ee5c2-047d-0010-87a3-c30de2ffd8ff.html
- Counterfeit Medicine Detection Using Blockchain and AI …, accessed July 28, 2025, https://oecd-opsi.org/innovations/counterfeit-medicine-detection-using-blockchain-and-ai/
- London Blockchain Startup FarmaTrust Partners with Mongolian Government to Stop Fake Medicine – Cointelegraph, accessed July 28, 2025, https://cointelegraph.com/press-releases/london-blockchain-startup-farmatrust-partners-with-mongolian-government-to-stop-fake-medicine
- OECD selects FarmaTrust’s Mongolia Project as Key Public Sector Trend – Medium, accessed July 28, 2025, https://medium.com/@farmatrust/oecd-selects-farmatrusts-mongolia-project-as-key-public-sector-trend-de64710e64ef
- Platform – Guardtime, accessed July 28, 2025, https://guardtime.com/platform
- Increasing Healthcare Security with Blockchain Technology …, accessed July 28, 2025, https://guardtime.com/blog/increasing-healthcare-security-with-blockchain-technology
- BlockRx – iSolve, accessed July 28, 2025, https://isolve.io/blockrx/
- health blockchain – Edinburgh Napier University, accessed July 28, 2025, https://www.napier.ac.uk/research-and-innovation/research-search/projects/health-blockchain
- Improving Medical Device Safety and Quality Using DLT/Blockchain …, accessed July 28, 2025, https://himsstv.brightcovegallery.com/detail/video/6033564629001/improving-medical-device-safety-and-quality-using-dlt-blockchain?autoStart=true&q=susan
- Medicalchain • Halston B2B, accessed July 28, 2025, https://www.halston.marketing/case-studies/medicalchain/
- Our Imperial / Better online | Be inspired, accessed July 28, 2025, https://www.imperial.ac.uk/be-inspired/magazine/issue-51/our-imperial–better-online/
- Competitive intelligence and patent analysis in drug discovery – PubMed, accessed July 28, 2025, https://pubmed.ncbi.nlm.nih.gov/24981938/
- Blockchain Patent Landscape: Comprehensive Guide for 2025 – Rahul Dev, accessed July 28, 2025, https://patentbusinesslawyer.com/blockchain-patent-landscape-comprehensive-guide-for-2024-and-2025/
- Blockchain patent landscaping: An expert based methodology and search query, accessed July 28, 2025, https://www.researchgate.net/publication/341800557_Blockchain_patent_landscaping_An_expert_based_methodology_and_search_query
- The basics of drug patent searching – DrugPatentWatch, accessed July 28, 2025, https://www.drugpatentwatch.com/blog/the-basics-of-drug-patent-searching/
- Patent Considerations for Blockchain in Medical Records, accessed July 28, 2025, https://patentpc.com/blog/patent-considerations-for-blockchain-in-medical-records
- How to Conduct a Drug Patent FTO Search – DrugPatentWatch, accessed July 28, 2025, https://www.drugpatentwatch.com/blog/how-to-conduct-a-drug-patent-fto-search/
- Leveraging Drug Patent Data for Strategic Investment Decisions: A …, accessed July 28, 2025, https://www.drugpatentwatch.com/blog/leveraging-drug-patent-data-for-strategic-investment-decisions-a-comprehensive-analysis/
- DrugPatentWatch Reviews – 2025 – Slashdot, accessed July 28, 2025, https://slashdot.org/software/p/DrugPatentWatch/
- (PDF) Analysing Opportunities and Challenges of Integrated Blockchain Technologies in Healthcare – ResearchGate, accessed July 28, 2025, https://www.researchgate.net/publication/327229059_Analysing_Opportunities_and_Challenges_of_Integrated_Blockchain_Technologies_in_Healthcare
- Patent Analysis – Evalueserve, accessed July 28, 2025, https://www.evalueserve.com/patent-analysis/
- Full article: Blockchain implementation in pharmaceutical supply chains: A review and conceptual framework – Taylor & Francis Online, accessed July 28, 2025, https://www.tandfonline.com/doi/full/10.1080/00207543.2022.2125595
- Using Blockchain to Drive Supply Chain Transparency and … – Deloitte, accessed July 28, 2025, https://www.deloitte.com/us/en/services/consulting/articles/blockchain-supply-chain-innovation.html
- Blockchain To Transform The Pharmaceutical Supply Chains: Drug Traceability and Authentication – GlobalRPH, accessed July 28, 2025, https://globalrph.com/2024/04/blockchain-in-pharmaceutical-supply-chain/
- Blockchain Healthcare | Interoperability using Blockchain – Wipro, accessed July 28, 2025, https://www.wipro.com/engineering/healthcare-data-interoperatibility-using-blockchain/
- IEEE 3205-2023 – IEEE SA, accessed July 28, 2025, https://standards.ieee.org/ieee/3205/10237/
- Overcoming Challenges of Blockchain Integration in Healthcare: A Comprehensive Analysis of Current Limitations | Simbo AI – Blogs, accessed July 28, 2025, https://www.simbo.ai/blog/overcoming-challenges-of-blockchain-integration-in-healthcare-a-comprehensive-analysis-of-current-limitations-3771045/
- How Pharmaceutical Blockchain Technology Challenges Can Be Overcome, accessed July 28, 2025, https://www.drugpatentwatch.com/blog/how-pharmaceutical-blockchain-technology-challenges-can-be-overcome/
- Identifying the Barriers to Acceptance of Blockchain-Based Patient-Centric Data Management Systems in Healthcare – PMC, accessed July 28, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC10855174/
- Full article: Evaluating the barriers of blockchain adoption in the Australian logistics industry, accessed July 28, 2025, https://www.tandfonline.com/doi/full/10.1080/17517575.2024.2397633?scroll=top&needAccess=true
- Challenges and Opportunities of Implementing Blockchain in Healthcare Systems, accessed July 28, 2025, https://www.researchgate.net/publication/391319870_Challenges_and_Opportunities_of_Implementing_Blockchain_in_Healthcare_Systems/download
- Regulatory Compliance for Blockchain – Number Analytics, accessed July 28, 2025, https://www.numberanalytics.com/blog/regulatory-compliance-blockchain-healthcare
- Blockchain Compliance in Healthcare – Number Analytics, accessed July 28, 2025, https://www.numberanalytics.com/blog/blockchain-compliance-healthcare
- The Role of Blockchain in Pharma – Medical Packaging Inc, accessed July 28, 2025, https://medpak.com/blockchain-pharmacy/
- How Artificial Intelligence & Blockchain Are Changing Drug Discovery – SCORR Marketing, accessed July 28, 2025, https://www.scorrmarketing.com/resources/ai-blockchain-primer/
- What is Blockchain and Artificial Intelligence (AI)? – IBM, accessed July 28, 2025, https://www.ibm.com/think/topics/blockchain-ai
- Enhancing Clinical Trials: The Impact of AI on Efficiency, Recruitment, and Data Analysis in Healthcare | Simbo AI – Blogs, accessed July 28, 2025, https://www.simbo.ai/blog/enhancing-clinical-trials-the-impact-of-ai-on-efficiency-recruitment-and-data-analysis-in-healthcare-3629263/
- Machine Learning and AI in Clinical Trials: Use Cases [2025] – Coherent Solutions, accessed July 28, 2025, https://www.coherentsolutions.com/insights/role-of-ml-and-ai-in-clinical-trials-design-use-cases-benefits
- Role of Artificial Intelligence in Clinical Trials and Healthcare Research – Appinventiv, accessed July 28, 2025, https://appinventiv.com/blog/artificial-intelligence-in-clinical-trials/
- Improving Participant Recruitment in Clinical Trials: Comparative Analysis of Innovative Digital Platforms – PubMed Central, accessed July 28, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC11694053/
- Applying Blockchain Technology to Enhance Clinical Trial Recruitment – PMC, accessed July 28, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC7153067/
- www.geeksforgeeks.org, accessed July 28, 2025, https://www.geeksforgeeks.org/ethical-hacking/blockchain-in-genomics/#:~:text=In%20conclusion%2C%20blockchain%20technology%20offers,in%20personalized%20medicine%20and%20research.
- Blockchain in Genomics – GeeksforGeeks, accessed July 28, 2025, https://www.geeksforgeeks.org/ethical-hacking/blockchain-in-genomics/
- Blockchain for genomics and healthcare: a literature review, current status, classification and open issues – PeerJ, accessed July 28, 2025, https://peerj.com/articles/12130/