The pharmaceutical industry operates under a stringent regulatory framework, with the development of new drugs requiring a multi-stage process overseen by governmental agencies. A critical juncture in this process is the submission and review of an Investigational New Drug (IND) application to the United States Food and Drug Administration (FDA).1 An IND serves as a formal request from a clinical study sponsor seeking authorization to administer an investigational drug or biological product to humans for the purpose of conducting clinical studies.1 This application is the mechanism through which a sponsor technically obtains an exemption from the federal law requiring an approved marketing application before a drug can be transported or distributed across state lines for clinical investigations.3 The primary objective during the early preclinical development phase under an IND is to ascertain whether the product is reasonably safe for initial use in humans and if it demonstrates sufficient pharmacological activity to warrant further commercial development.3
Complementary to the regulatory oversight of drug development is the principle of governmental transparency, primarily facilitated by the Freedom of Information Act (FOIA).4 Enacted to ensure public access to information held by federal agencies, FOIA provides the public with a statutory right to request and obtain records from any federal agency, including the FDA.4 This right is predicated on the notion that an informed citizenry is vital to the functioning of a democratic society.6 Federal agencies are obligated to disclose requested information unless it falls within one of nine specific exemptions that protect interests such as national security, personal privacy, and trade secrets.5 This report aims to analyze the accessibility of IND applications and their contents through the lens of the Freedom of Information Act. It will examine the legal framework governing such access, focusing on the interplay between FOIA’s disclosure mandate and the specific regulations and exemptions that pertain to the sensitive information contained within INDs. Furthermore, the report will explore the practical considerations for individuals or organizations seeking to obtain IND information, highlighting the challenges and limitations inherent in this process, while also considering the balance between protecting proprietary information and serving the broader public interest in accessing data related to drug development.
The legal framework governing the accessibility of Investigational New Drug Applications is shaped by the Freedom of Information Act, a cornerstone of government transparency in the United States.4 FOIA generally grants any person, regardless of their citizenship, the right to request access to agency records held by federal entities.5 As a federal agency within the Department of Health and Human Services responsible for regulating drugs and biological products, the FDA is subject to the provisions of FOIA.9 The FDA has established its own dedicated FOIA office and specific procedures for managing and responding to information requests from the public.10
While FOIA establishes a broad principle of disclosure, it also recognizes that certain types of information warrant protection from public scrutiny. To this end, the Act includes nine specific exemptions that permit federal agencies to withhold requested information under defined circumstances.5 Among these exemptions, Exemption 4 is particularly relevant to the context of IND applications. This exemption protects “trade secrets and commercial or financial information obtained from a person that is privileged or confidential”.4 The underlying rationale for Exemption 4 is to safeguard the competitive interests of businesses that provide information to the government, as well as to ensure the government’s ability to obtain reliable commercial and financial data.15
In the realm of pharmaceutical development, the FDA and drug manufacturers frequently invoke Exemption 4 as the basis for withholding the disclosure of IND applications and their contents.4 The justification for this stance is that INDs typically contain a wealth of information that qualifies as trade secrets and confidential commercial information, the disclosure of which could potentially cause significant harm to the drug sponsor’s competitive standing.4 This commercially sensitive information within an IND can include details about the drug’s structural formula, the results of animal testing, the intricate processes involved in its manufacturing, and the protocols governing proposed clinical trials.3 The FDA has historically maintained that even acknowledging the mere existence of an IND could be detrimental to a drug sponsor’s competitive position in the market.4 It is important to note that the legal interpretation of what constitutes “confidential” commercial information under Exemption 4 has varied across different federal circuit courts, adding a layer of complexity to the application of this exemption in specific cases.20
Beyond the general framework of FOIA, the FDA has promulgated specific regulations addressing the public disclosure of data and information contained within Investigational New Drug Applications. Title 21, Section 312.130 of the Code of Federal Regulations (21 CFR § 312.130) directly governs the availability of public disclosure for INDs.4 This regulation establishes a fundamental principle: the FDA will not disclose the existence of an IND unless that existence has been previously made public or officially acknowledged.4
Furthermore, 21 CFR § 312.130 delineates how the disclosure of information within an IND is to be handled, depending on whether the investigational product is a new drug or a biological product. For new drugs, the regulation explicitly states that the availability for public disclosure of all data and information in an IND will be managed in accordance with the provisions established in 21 CFR § 314.430, which pertains to the confidentiality of data and information in New Drug Applications (NDAs) submitted under Part 314 of the FDA’s regulations.21 Similarly, for investigational new drug applications concerning biological products, the rules governing public disclosure are those outlined in 21 CFR §§ 601.50 and 601.51, which address the confidentiality of information within Biologics License Applications (BLAs).21
The cross-reference to 21 CFR § 314.430 is particularly significant for understanding the FDA’s approach to IND disclosure. This regulation outlines the agency’s policy on the public availability of any data or information submitted as part of a drug application, including INDs.21 A key provision of § 314.430 is that if the existence of an unapproved application has not been publicly disclosed or acknowledged, then no data or information contained within that application is available for public disclosure.22 However, once an NDA is approved and the FDA sends an approval letter to the applicant, certain categories of safety and effectiveness data, along with summaries of this data and related correspondence, may become available for public disclosure, although this is subject to the various exemptions предусмотренные in FOIA and FDA regulations.22 While the provided snippets do not detail the specific provisions of 21 CFR §§ 601.50 and 601.51 regarding BLAs, the principle of linking IND disclosure to the regulations governing the marketing application suggests a similar approach to managing the confidentiality of information for biological products.
Notwithstanding these general rules of confidentiality, 21 CFR § 312.130 also specifies certain circumstances under which the FDA is mandated to disclose information from an IND. Specifically, the regulation states that the FDA must disclose, upon request, a copy of any IND safety report that relates to the use of the investigational new drug by the individual making the request, regardless of the provisions in § 314.430.21 This provision underscores the importance of ensuring that individuals who have received an investigational drug have access to information concerning its safety. Furthermore, for clinical investigations that have been granted an exception from the requirement of obtaining informed consent under 21 CFR § 50.24 (which typically pertains to emergency research situations), the availability of publicly disclosable information from the IND is handled through the Freedom of Information Act. Individuals wishing to request this specific information, which was initially filed in Docket Number 95S-1061 (later referenced as 95S-0158), must submit a FOIA request to the FDA’s Dockets Management Staff.21 These exceptions highlight a regulatory recognition of the individual’s right to know about their own safety and the heightened need for transparency when standard ethical safeguards are modified in research settings.
The FDA’s general policy is to refrain from disclosing the existence or the contents of IND applications that have not yet received marketing approval.4 This stance is primarily driven by the concern that the premature release of such information could significantly undermine the competitive advantage of the drug sponsor.4 The agency operates under the understanding that INDs contain sensitive details about the drug’s development, and public knowledge of these details could be exploited by competitors.
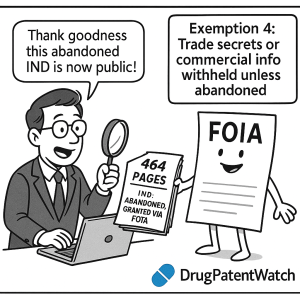
However, the landscape of IND information accessibility shifts somewhat in the context of abandoned or inactive applications. Regulations allow for an IND to be placed in an inactive status by either the sponsor or the FDA if no subjects have been enrolled in clinical studies for a period of two years or more, or if the investigation remains on clinical hold for at least one year.23 An IND can be formally withdrawn by the applicant if the development of the investigational product is abandoned for any reason.23 In such cases, certain data contained within the abandoned IND may become accessible through a FOIA request, particularly information pertaining to the safety and effectiveness of the drug.4 A notable example of this is the case of Hoffman-La Roche’s abandoned IND for Ro 24-7429. In response to a specific FOIA request, the FDA provided the researchers with the IND application number and 464 pages of relevant material. This included the agency’s initial pharmacology review and an annual report that contained an investigational drug brochure, clinical study protocols and amendments.4 The researchers who successfully obtained this information believed that the data was no longer of commercial interest to the company, and therefore, its public disclosure would not cause any private harm.4 This case suggests that the “abandoned” status of an IND can be a crucial factor in potentially gaining FOIA access, indicating that the FDA’s concern for competitive harm may diminish over time. However, it is important to note that the researchers’ experience was described as being “contrary to others’ experience,” suggesting that successful access to abandoned INDs is not always guaranteed and may depend on the specific circumstances of the request and the information sought.4
Once a drug successfully navigates the clinical trial process and receives marketing approval, the landscape of information accessibility changes significantly. After an NDA or BLA is approved, information related to the approval may be released under FOIA.17 The standard information package associated with the approval of a New Drug Application typically includes the official approval letter, the drug’s package insert and labeling, the FDA’s final review documents, and a Summary Basis of Approval (SBA).17 The SBA is a particularly valuable document as it contains a summary of the safety and effectiveness data and information that was evaluated by the FDA during the drug approval process.22 While it is worth noting that SBAs are no longer prepared for most new drug approvals, the same essential information is generally made available through the FDA’s comprehensive review documents, albeit in a different format.17 Given that the IND phase lays the foundational preclinical and early clinical data that ultimately informs the NDA review, the information disclosed post-NDA approval, such as the SBA and FDA reviews, can provide valuable retrospective insights into the data and assessments that were generated during the earlier IND phase of development. This suggests that while direct access to active INDs remains highly restricted, the conclusions and key findings derived from the information contained within them become more transparent to the public once the drug is deemed safe and effective and reaches the market.
For individuals or organizations seeking to request information from an Investigational New Drug Application via the Freedom of Information Act, a structured approach is essential. The first step is to submit a written FOIA request to the FDA, ensuring that it includes specific required information.5 This request must clearly state the requester’s full name, mailing address, and a telephone number where they can be reached.5 Crucially, the request should contain a clear and detailed description of the specific records being sought, identified as precisely as possible.5 Providing specific details, such as the name of the drug, the IND application number (if known), the timeframe of interest, and the particular types of documents or data being requested, can significantly enhance the FDA’s ability to process the request efficiently.13 Conversely, overly broad or vague requests for “all information” on a particular subject are less likely to yield timely results and may incur higher fees.13 The written request should also include a statement indicating the requester’s willingness to pay any associated fees for the processing of the request, and if applicable, it should specify any limitations on the maximum amount the requester is willing to pay.13
The FDA offers several methods for submitting FOIA requests. While the agency prefers electronic submissions through its online portal, accessible at(http://www.accessdata.fda.gov/scripts/foi/FOIRequest/index.cfm) or via the broader FOIA.gov website, requests can also be submitted via mail to the FDA’s Division of Headquarters Freedom of Information in Rockville, Maryland.12 It is important to note that the FDA is currently not accepting FOIA requests submitted via facsimile.13
It is also important to be aware of the potential fees associated with submitting a FOIA request to the FDA.4 While there is no initial fee to submit a request, the agency may charge fees for the costs incurred in searching for, reviewing, and duplicating the requested records.4 However, the FOIA does provide for the possibility of obtaining a fee waiver or reduction in certain circumstances.5 Specifically, a fee waiver may be granted if the requester can demonstrate that the disclosure of the requested information is in the public interest because it is likely to contribute significantly to the public’s understanding of the operations or activities of the government, and the disclosure is not primarily in the commercial interest of the requester.5 When submitting a FOIA request, it is advisable to indicate a maximum dollar amount the requester is willing to pay for the processing of the request.13
Under the Freedom of Information Act, federal agencies are generally required to make a determination regarding a FOIA request within 20 working days of its receipt.32 However, the FDA, like other agencies, may request a 10-working-day extension if necessary, and in cases involving particularly complex requests, they may negotiate a mutually agreeable response timeframe with the requester, especially if the process is expected to take longer than 30 working days.32 Despite these statutory guidelines, the FDA, facing a high volume of FOIA requests annually, has sometimes experienced significant delays in responding to requests, with average response times occasionally exceeding 200 days.32 These delays can be attributed to various factors, including the sheer volume of requests received, the complexity of the information sought (which may necessitate extensive searches across numerous records), the need for thorough review and redaction of documents to protect confidential commercial information and other exempt material, and limitations in the agency’s resources.32 Therefore, while the statutory timeline provides a benchmark, requesters should be prepared for the possibility of delays, particularly when seeking extensive or sensitive information like that contained in IND applications.
Despite the principles of transparency embodied in the Freedom of Information Act, accessing information contained within Investigational New Drug Applications often presents significant challenges. The primary reason for the FDA’s denial of FOIA requests for IND information is the application of Exemption 4, which protects trade secrets and confidential commercial information.4 The FDA consistently maintains that the information contained within an IND is highly sensitive from a commercial perspective, and even acknowledging the existence of an IND could potentially harm the drug sponsor’s competitive position in the market.4
The terms “trade secret” and “confidential commercial information” are central to the application of Exemption 4 in the context of pharmaceutical development. A trade secret can encompass a wide range of proprietary information, including a formula, practice, process, design, instrument, or compilation of information that provides a business with a competitive edge over others who do not know or use it.4 Confidential commercial information is generally understood to be information that, if disclosed, would likely cause substantial harm to the competitive position of the entity that submitted it.15 Pharmaceutical companies invest vast amounts of time, capital, and resources in the research and development of new drugs, making the detailed scientific, manufacturing, and clinical trial data contained within INDs exceptionally valuable and, in their view, warranting robust protection under Exemption 4.18
This protection of commercial interests, however, often stands in tension with the potential public health benefits that could arise from greater transparency in the drug development process.4 Advocates for increased access to IND information argue that making such data available, particularly for drugs that have been abandoned or have raised safety concerns, could serve the public interest by facilitating drug repurposing efforts for other diseases and by informing crucial treatment decisions for healthcare professionals and patients.4 Conversely, drug sponsors often contend that the premature disclosure of information contained in INDs could undermine their significant investments, stifle innovation by allowing competitors to benefit from their research, and ultimately reduce the incentive to engage in the costly and high-risk process of drug development.18 The FDA’s current regulatory approach generally reflects a prioritization of protecting the commercial interests of drug sponsors during the investigational phase, with a gradual increase in transparency occurring after a drug receives marketing approval.4 This balance between commercial protection and public health transparency remains a subject of ongoing debate and scrutiny in the pharmaceutical regulatory landscape.
Examining specific instances where FOIA requests have been made for IND information provides valuable context for understanding the practical realities of accessing this data. The successful endeavor by researchers to obtain the abandoned IND of Hoffman-La Roche for Ro 24-7429 offers a significant case study.4 Their request yielded 464 pages of documentation, including insightful pharmacology reviews conducted by the FDA and comprehensive annual reports from the sponsor.4 The researchers attributed their success to the fact that the IND had been abandoned and their request was specifically tailored to safety and effectiveness data, allowing them to argue that the information was no longer commercially sensitive and held potential value for public health, particularly in the context of drug repurposing initiatives.4
However, the very account of this successful request notes that it was “contrary to others’ experience,” highlighting the general difficulty in obtaining IND information through FOIA.4 Another illustrative case involves the Public Citizen Health Research Group’s efforts to access information from Shering’s IND 18113 for labetalol.4 This instance required not only an initial FOIA request but also a subsequent lawsuit and appeal to the agency, ultimately resulting in only a “partial judgment in favor of IND data release”.4 This suggests that the FDA’s default position is often to withhold IND information, and requesters may need to be prepared for a potentially protracted and legally challenging process. Reinforcing this point is the general statement found in one of the research snippets indicating that “Except in rare circumstances, documents regarding an IND are not disclosable under the Freedom of Information Act”.17 This underscores the significant hurdles that typically exist when attempting to gain access to IND-related documents. While FOIA logs of various government agencies might contain further examples of requests pertaining to pharmaceutical information, the provided snippets do not offer additional specific case studies of successful or unsuccessful IND requests beyond the Ro 24-7429 and labetalol examples. However, the overall narrative from these cases suggests that successful FOIA requests for INDs are often contingent upon specific circumstances, such as the abandoned status of the drug and a narrowly focused request that minimizes concerns about the disclosure of commercially sensitive information while emphasizing the potential for public benefit.
In conclusion, accessing information from Investigational New Drug Applications through the Freedom of Information Act is a complex endeavor with significant limitations. The FDA generally restricts access to IND information, primarily relying on FOIA Exemption 4 to protect what it considers trade secrets and confidential commercial information. The agency typically does not disclose the existence of an IND unless it has already been made public. However, in rare instances, access may be granted, particularly for INDs that have been abandoned or are no longer active, especially when the request is specific and focuses on safety and effectiveness data that may no longer hold substantial commercial value. Obtaining such information often requires a compelling justification for public interest and may necessitate considerable persistence, and in some cases, legal action. Conversely, information related to drugs that have received marketing approval (NDAs/BLAs) becomes more accessible post-approval through documents like the Summary Basis of Approval and FDA review documents, offering retrospective insights into the data initially submitted during the IND phase.
Based on the analysis, the likelihood of successfully obtaining different types of IND information through FOIA varies considerably:
| Type of IND Information | Likelihood of FOIA Access | Key Considerations |
| Information from active INDs | Very Low | Strong protection under Exemption 4 (trade secrets and confidential commercial information). FDA generally does not even acknowledge existence. |
| Detailed manufacturing processes/chemical formulas | Low | Highly likely to be considered trade secrets, even for abandoned INDs. |
| Safety and effectiveness data from abandoned/inactive INDs | Moderate | Higher chance if the request is specific, focuses on these data points, and argues that the information is no longer commercially sensitive and has public health value (e.g., for repurposing). Success is not guaranteed. |
| Existence of an IND | Very Low | FDA generally does not confirm unless publicly disclosed or acknowledged by the sponsor. |
| IND safety reports related to individual use | High | Mandated disclosure to the individual who received the investigational drug upon request. |
| Information related to exceptions from informed consent | Moderate | Specific FOIA pathway exists for information filed in Docket Number 95S-0158. |
| Information about approved drugs (post-NDA/BLA) | High | Summary Basis of Approval (SBA), FDA reviews, and other approval-related documents are generally available under FOIA, offering insights into the data that originated in the IND phase. |
For individuals or organizations contemplating a FOIA request for IND information, the following recommendations are offered:
- Prior to submitting a request, conduct thorough research to determine if the desired information is already publicly available through clinical trial registries, scientific publications, or FDA databases related to approved drugs.4
- Ensure the FOIA request is as specific as possible, clearly identifying the drug, the IND application number (if known), the precise types of documents or data being sought, and the relevant timeframe.13
- When seeking information from an abandoned IND, explicitly articulate the reasons for believing the information is no longer commercially sensitive and emphasize the potential public interest or public health benefits that could result from its disclosure.4
- Recognize the significant protections afforded by FOIA Exemption 4 and be prepared for the possibility of the request being denied based on the need to safeguard trade secrets and confidential commercial information.4
- Adhere strictly to the FDA’s established FOIA request procedures, including utilizing the preferred online submission portal or sending the request to the correct mailing address.12
- Include a clear statement regarding the requester’s willingness to pay processing fees and consider requesting a fee waiver if the disclosure aligns with the public interest criteria outlined in FOIA.5
- Anticipate potential delays in receiving a response from the FDA and be prepared to follow up on the request if the statutory response timelines are exceeded.32
- Understand the process for appealing a FOIA denial and consider pursuing an appeal if there are valid grounds to believe the denial was not justified.5
- Individuals who have participated in a clinical trial under an IND should remember their right to request and receive IND safety reports related to their specific use of the investigational drug.21
The stringent limitations on FOIA access to active INDs underscore the significant emphasis placed on protecting the intellectual property and competitive advantages of pharmaceutical companies during the crucial stages of research and development. Conversely, the occasional success in obtaining information from abandoned INDs suggests a potential pathway for post-development transparency, recognizing that the commercial sensitivity of such information may diminish over time, while its public health value could persist.
Works cited
- Investigational New Drug Applications (INDs) for CBER-Regulated Products | FDA, accessed May 8, 2025, https://www.fda.gov/vaccines-blood-biologics/development-approval-process-cber/investigational-new-drug-applications-inds-cber-regulated-products
- Investigational New Drug (IND) Application | NIH – Clinical Info HIV.gov, accessed May 8, 2025, https://clinicalinfo.hiv.gov/en/glossary/investigational-new-drug-ind-application
- Investigational New Drug (IND) Application – FDA, accessed May 8, 2025, https://www.fda.gov/drugs/types-applications/investigational-new-drug-ind-application
- Freedom of Information Act Access to an Investigational New Drug Application – PMC, accessed May 8, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC7089016/
- Freedom of Information Act: Frequently Asked Questions (FAQ) – FOIA.gov, accessed May 8, 2025, https://www.foia.gov/faq.html
- FOIA.gov – Freedom of Information Act, accessed May 8, 2025, https://www.foia.gov/
- The Freedom of Information Act (FOIA): A Legal Overview | Congress.gov, accessed May 8, 2025, https://www.congress.gov/crs-product/R46238
- Food and Drug Administration (FDA) Information Disclosure Manual, February 1999 – Government Attic, accessed May 8, 2025, https://www.governmentattic.org/6docs/FDA-InfoDiscManual_1999.pdf
- Investigational New Drug Applications; Exemptions for Clinical Investigations To Evaluate a Drug Use of a Product Lawfully Marketed as a Conventional Food, Dietary Supplement, or Cosmetic – Federal Register, accessed May 8, 2025, https://www.federalregister.gov/documents/2022/12/09/2022-26728/investigational-new-drug-applications-exemptions-for-clinical-investigations-to-evaluate-a-drug-use
- Freedom of Information – FDA, accessed May 8, 2025, https://www.fda.gov/regulatory-information/freedom-information
- OII FOIA Electronic Reading Room – FDA, accessed May 8, 2025, https://www.fda.gov/about-fda/office-inspections-and-investigations/oii-foia-electronic-reading-room
- Frequently Asked Questions (FAQ) for Freedom of Information – FDA, accessed May 8, 2025, https://www.fda.gov/regulatory-information/freedom-information/frequently-asked-questions-faq-freedom-information
- How to Make a FOIA Request | FDA, accessed May 8, 2025, https://www.fda.gov/regulatory-information/freedom-information/how-make-foia-request
- FDA Freedom of Information Act (FOIA), accessed May 8, 2025, https://www.accessdata.fda.gov/scripts/foi/foirequest/requestinfo.cfm
- Exemption 4 – Department of Justice, accessed May 8, 2025, https://www.justice.gov/archive/oip/foia_guide09/exemption4.pdf
- FOIA Guide, 2004 Edition: Exemption 4 – Department of Justice, accessed May 8, 2025, https://www.justice.gov/archives/oip/foia-guide-2004-edition-exemption-4
- Drug Documents – FOI Services, accessed May 8, 2025, https://www.foiservices.com/brochure/drugdocs.cfm
- Disclosure of Clinical Trial Data: Why Exemption 4 of the Freedom of Information Act Should Be Restored – Duke Law Scholarship Repository, accessed May 8, 2025, https://scholarship.law.duke.edu/cgi/viewcontent.cgi?article=1123&context=dltr
- Sometimes the Silence Can Be like the Thunder: Access to Pharmaceutical Data at the FDA, accessed May 8, 2025, https://scholarship.law.duke.edu/cgi/viewcontent.cgi?article=1388&context=lcp
- Protecting Trade Secrets Disclosed To The FDA – Skadden, Arps, Slate, Meagher & Flom LLP, accessed May 8, 2025, https://www.skadden.com/-/media/files/publications/2018/02/protecting_trade_secrets_disclosed_to_the_fda.pdf
- 21 CFR 312.130 — Availability for public disclosure of data and information in an IND. – eCFR, accessed May 8, 2025, https://www.ecfr.gov/current/title-21/chapter-I/subchapter-D/part-312/subpart-F/section-312.130
- 21 CFR § 314.430 – Availability for public disclosure of data and …, accessed May 8, 2025, https://www.law.cornell.edu/cfr/text/21/314.430
- IND Application Procedures: Investigator’s Responsibilities – FDA, accessed May 8, 2025, https://www.fda.gov/drugs/investigational-new-drug-ind-application/ind-application-procedures-investigators-responsibilities
- IND Maintenance – NIH Clinical Center, accessed May 8, 2025, https://www.cc.nih.gov/orcs/ind/maintenance
- Freedom of Information Act Access to an Investigational New Drug Application – PubMed, accessed May 8, 2025, https://pubmed.ncbi.nlm.nih.gov/32259081/
- Freedom of Information Act Access to an Investigational New Drug Application | ACS Pharmacology & Translational Science – ACS Publications, accessed May 8, 2025, https://pubs.acs.org/doi/abs/10.1021/acsptsci.9b00056
- Freedom of Information Act Access to an Investigational New Drug Application, accessed May 8, 2025, https://www.drugpatentwatch.com/blog/freedom-of-information-act-access-to-an-investigational-new-drug-application/
- Food and Drug Administration Center for Drugs and Biologics – Medical Technology Assessment Directory – NCBI, accessed May 8, 2025, https://www.ncbi.nlm.nih.gov/books/NBK218390/
- FOIA and Privacy Act FAQs – USPTO, accessed May 8, 2025, https://www.uspto.gov/learning-and-resources/general-help/foia-and-privacy-act-faqs
- FDA FOIA Request Form, accessed May 8, 2025, https://www.accessdata.fda.gov/scripts/foi/foirequest/requestform.cfm
- Sample FOIA Request Letters – National Freedom of Information Coalition, accessed May 8, 2025, https://www.nfoic.org/sample-foia-request-letters/
- FDA FOIA Requests: A Deeper Look into Key Challenges and Stats – Logikcull, accessed May 8, 2025, https://www.logikcull.com/blog/fda-foia-requests
- More on the Impact of the FDA RIFs: How Information Disclosure will Start FOIA-lling Behind, accessed May 8, 2025, https://www.thefdalawblog.com/2025/04/more-on-the-impact-of-the-fda-rifs-how-information-disclosure-will-start-foia-lling-behind/
- Comments on FDA Transparency – Public Citizen, accessed May 8, 2025, https://www.citizen.org/article/comments-on-fda-transparency/
- Frequently Asked Questions | ClinicalTrials.gov, accessed May 8, 2025, https://clinicaltrials.gov/policy/faq
- FOIA Requests and Appeals | U.S. Equal Employment Opportunity Commission, accessed May 8, 2025, https://www.eeoc.gov/foia/submit-request-or-appeal