Executive Summary
The engagement of a Contract Development and Manufacturing Organization (CDMO) has fundamentally evolved from a tactical cost-saving measure into a core strategic function essential for competitive advantage in the pharmaceutical and biotechnology industries. The decision to outsource is no longer a simple “build vs. buy” calculation; it is a critical lever for accelerating time-to-market, accessing cutting-edge innovation, and building resilient global supply chains. This report provides a comprehensive playbook for navigating the complexities of the CDMO landscape, from initial market assessment to long-term partnership management.
The CDMO market is undergoing a period of unprecedented expansion, projected to grow from approximately USD 255 billion in 2025 to over USD 465 billion by 2032.1 This growth is supercharged by the rise of complex biologics, cell and gene therapies, and geopolitical forces like the US BIOSECURE Act, which are actively reshaping global supply chains.2 In this dynamic environment, success hinges on a holistic, disciplined approach to partnership.
This guide demonstrates that a successful collaboration is built upon three foundational pillars. First, a rigorous and data-driven selection process is paramount. This involves a meticulous internal needs assessment, a multi-domain due diligence process that scrutinizes technical, quality, operational, and financial capabilities, and the strategic use of novel tools like patent intelligence to validate a potential partner’s true expertise. Second, the partnership must be architected on robust legal and contractual frameworks. The Master Service Agreement (MSA) and Quality Agreement (QA) are not boilerplate documents but strategic instruments for allocating risk, defining responsibilities, and, most critically, protecting a company’s intellectual property (IP) to ensure future operational freedom. Finally, the collaboration must be executed through a proactive governance model built on the principle of “trust but verify”.4 This involves establishing joint project management structures, executing flawless technology transfers, and integrating supply chains, all while having clear mechanisms for managing risk and resolving conflict.
The stakes of failure are immense. A poor CDMO partnership can lead to catastrophic financial losses, crippling regulatory setbacks, and reputational damage. However, the most significant cost is often the irreversible loss of time—delaying a drug’s market entry erodes its valuable period of patent exclusivity, a strategic cost that can dwarf all other financial considerations.5 This report equips executive leaders and operational teams with the market intelligence, strategic frameworks, and actionable recommendations necessary to mitigate these risks and forge the durable, high-performing CDMO partnerships that are now indispensable for success.
Section 1: The Modern CDMO Landscape: A Strategic Overview
The decision to partner with a Contract Development and Manufacturing Organization (CDMO) is no longer a peripheral operational choice but a central component of corporate strategy for pharmaceutical and biotechnology companies of all sizes. Understanding the dynamics of this rapidly evolving market is the first step toward leveraging these partnerships effectively. The sector is characterized by explosive growth, a fundamental shift in the strategic rationale for outsourcing, significant industry consolidation, and the emergence of disruptive technological and geopolitical trends.
1.1 Market Dynamics and Growth Trajectory
The CDMO market is experiencing a period of remarkable and sustained growth, reflecting the industry’s increasing reliance on external partners. The global pharmaceutical CDMO market is projected to expand from a value of USD 255.01 billion in 2025 to USD 465.24 billion by 2032, which represents a compound annual growth rate (CAGR) of 9.0%.1 Other market analyses corroborate this strong upward trend, with estimates projecting a global market size of USD 368.7 billion by 2034, growing at a CAGR of approximately 6.9%.7
This expansion is not uniform across all market segments. Certain areas are growing at a significantly faster pace, driven by the increasing complexity of modern therapeutics:
- Biologics: The biologics CDMO market is a key engine of this growth. One forecast predicts this segment will grow by USD 16.32 billion between 2025 and 2029, accelerating at an impressive CAGR of 13.7%.2 This is fueled by the robust pipeline of monoclonal antibodies, vaccines, cell therapies, and gene therapies, which require highly specialized manufacturing capabilities that are difficult and expensive to establish in-house.2 Mammalian cell culture systems, essential for producing many biologics like monoclonal antibodies, commanded over 62% of the biologics CDMO market in 2024.9
- Investigational New Drug (IND) Support: The market for CDMOs supporting investigational new drugs—a direct indicator of R&D pipeline activity—is also poised for substantial growth. It is forecast to grow from USD 5.61 billion in 2025 to USD 10.26 billion by 2034, expanding at a CAGR of 6.93%.10 This reflects the increasing number of clinical trials and the outsourcing of early-stage development and clinical trial material manufacturing.10
Geographically, the market exhibits a clear division between established dominance and rapid emergence. North America, particularly the U.S., currently holds the largest share of the CDMO market revenue, accounting for 44% of the investigational new drug CDMO market in 2024.9 However, the Asia-Pacific region is universally recognized as the fastest-growing market.8 Countries like China and India are becoming key players, driven by cost-efficient services, expanding pharmaceutical industries, and increasingly favorable regulatory environments that attract international investment.7
This robust, multi-faceted growth underscores a fundamental industry shift. The demand for CDMO services is not a temporary trend but a structural change in how pharmaceutical products are developed and manufactured. For sponsors, this means that securing reliable, high-quality CDMO capacity, especially in high-growth areas like biologics, is becoming an increasingly competitive and strategically critical endeavor.
1.2 The Strategic Rationale for Outsourcing: Beyond Cost-Efficiency
While cost savings were an early driver for outsourcing, the contemporary strategic rationale has evolved to encompass a much broader set of value propositions that are critical for competitive advantage. The modern impetus for partnering with a CDMO is less about reducing line-item expenses and more about acquiring strategic capabilities that accelerate growth and mitigate risk.
The primary driver is now speed-to-market. The journey to bring a new drug to market is an arduous and expensive one, typically taking 10 to 15 years and costing an average of USD 2.6 billion.5 CDMOs, with their specialized expertise, established facilities, and streamlined processes, can significantly shorten development and manufacturing timelines.12 This acceleration is not merely an operational efficiency; it is a profound strategic advantage. Every day saved in development is a day gained on the market under patent protection, directly increasing the period of market exclusivity and maximizing the revenue potential of an asset.5
A second critical driver is access to specialized expertise and technology. The pharmaceutical pipeline is increasingly dominated by complex modalities like cell and gene therapies, antibody-drug conjugates (ADCs), and mRNA vaccines.2 Developing the in-house infrastructure and human capital to manufacture these products requires prohibitive capital investment and a long lead time.12 CDMOs offer immediate access to state-of-the-art facilities, advanced technologies such as continuous manufacturing and single-use systems, and highly experienced scientific teams.5 This allows sponsors, particularly small and mid-sized biotech firms with limited or no internal manufacturing infrastructure, to compete on a more level playing field by leveraging the CDMO’s established capabilities.7
For large pharmaceutical companies, outsourcing serves as a vital tool for risk management and strategic flexibility. By partnering with CDMOs, they can de-risk their supply chains, creating redundancy and avoiding over-reliance on a single internal facility.7 It also provides the operational agility to scale production up or down in response to fluctuating market demand without the fixed costs and constraints of captive facilities.14 The global response to the COVID-19 pandemic provided a powerful case study in this regard, as companies like Moderna successfully partnered with a network of CDMOs to rapidly scale up global production of their mRNA vaccine, a feat that would have been impossible using only internal resources.12
1.3 Industry Consolidation and Key Players
The CDMO market, while still containing numerous specialized players, is undergoing a significant wave of consolidation.11 This trend is driven by larger CDMOs seeking to build integrated, end-to-end service platforms, often referred to as “one-stop-shops,” capable of guiding a client’s product from early-stage development through to commercial launch and packaging.7 This strategy is fueled by mergers and acquisitions (M&A), as demonstrated by recent deals such as the merger of BioCina and NovaCina to create an end-to-end global CDMO, and Thermo Fisher Scientific’s landmark acquisition of Patheon to form a powerhouse in the sector.9
This consolidation has given rise to a cadre of dominant global players who offer a comprehensive range of services across diverse modalities and geographies. Key industry leaders include:
- Lonza: A Swiss multinational and one of the largest CDMOs globally, with deep expertise in biologics, small molecules, and cell and gene therapies.16
- Catalent, Inc.: A U.S.-based leader known for its advanced drug delivery technologies, biologics development, and fill-finish capabilities.16
- Thermo Fisher Scientific (Patheon): A major force in the market offering integrated services from API and drug product development to clinical trial logistics and commercial manufacturing.16
- Boehringer Ingelheim: A major German pharmaceutical company with a significant biopharmaceutical contract manufacturing business.18
- WuXi Biologics: A leading global CDMO based in China, offering end-to-end solutions for biologics discovery, development, and manufacturing.9
A recent, landscape-altering development is the acquisition of Catalent by Novo Holdings, the parent company of Novo Nordisk, for USD 16.5 billion.3 A key part of this deal involves Novo Nordisk acquiring three of Catalent’s fill-finish manufacturing sites for USD 11.5 billion, presumably to secure capacity for its blockbuster GLP-1 drugs, Wegovy and Ozempic.3 This move highlights a new dynamic where unprecedented demand for a specific class of drugs can lead a major pharmaceutical company to vertically integrate by acquiring significant CDMO assets, effectively removing that capacity from the open market and intensifying competition for the remaining fill-finish services.
1.4 Emerging Trends Redefining the Sector
Beyond market growth and consolidation, several powerful trends are reshaping the strategic landscape of CDMO partnerships.
- Technology and Artificial Intelligence (AI): The integration of digital technologies is moving from a theoretical advantage to a practical reality. CDMOs are increasingly leveraging AI and big data for process optimization, predictive maintenance on equipment, and enhanced quality control systems.2 The goal is to use historical manufacturing data and machine learning algorithms to improve efficiency, forecast potential issues, and reduce costly manufacturing downtime.7 Furthermore, advanced modeling tools like “digital twins” are being used to simulate and de-risk complex processes like technology transfers and facility retrofits before any physical work begins, saving significant time and resources.19
- Geopolitical Influence and Supply Chain Regionalization: Geopolitical factors have become a critical variable in CDMO strategy. The proposed US BIOSECURE Act, which aims to prohibit U.S. government agencies from contracting with certain Chinese biomanufacturing companies like WuXi AppTec, is a major disruptive force.3 Regardless of its final legislative form, the act has already triggered a widespread re-evaluation of supply chain risk among Western pharmaceutical companies.3 This is accelerating a trend toward supply chain diversification and regionalization, creating significant opportunities for CDMOs in other regions, particularly India, who are positioning themselves to capture business shifting away from China.3 This demonstrates that a CDMO’s geographic location is no longer just a logistical consideration but a matter of long-term strategic security.
- Specialization vs. Integration: The market is bifurcating. On one hand, the consolidation trend is creating large, integrated “one-stop-shop” CDMOs. On the other hand, the sheer complexity of novel therapeutics is fueling the rise of highly specialized “boutique” CDMOs that offer deep, world-class expertise in a specific niche, such as cell therapy manufacturing, topical drug formulation, or lipid nanoparticle (LNP) delivery systems.2 This presents sponsors with a critical strategic choice at the outset of a project: opt for the simplified management of a single integrated partner or pursue the specialized excellence of a multi-vendor “best-of-breed” approach.22
The confluence of these market forces means that the decision to engage a CDMO is more complex and more strategic than ever before. It is a decision that directly impacts a company’s financial performance, its ability to innovate, and its resilience in an increasingly unpredictable global environment.
| Market Segment | 2025 Forecast (USD) | Projected Value | Forecast Period | CAGR | Key Drivers |
| Global Pharmaceutical CDMO | $255.01 Billion 1 | $465.24 Billion 1 | 2025-2032 | 9.0% 1 | Rising outsourcing, cost efficiency, focus on core competencies. |
| Investigational New Drug (IND) CDMO | $5.61 Billion 10 | $10.26 Billion 10 | 2025-2034 | 6.93% 10 | Increasing number of clinical trials, demand for biologics, R&D spending. |
| Biologics CDMO | $25.32 Billion 9 | $36.51 Billion 9 | 2025-2030 | 7.59% 9 | Complexity of next-gen therapies, demand for outsourced capacity. |
| Biologics CDMO (Alternate Forecast) | N/A | +$16.32 Billion Growth 2 | 2025-2029 | 13.7% 2 | Cost-efficient resources in emerging markets, demand for vaccines & immunotherapies. |
| API Manufacturing | N/A (70.68% of market in 2024) 11 | N/A | 2025-2030 | N/A | Criticality of API production, rising outsourcing trends, regulatory compliance needs. |
| North America Region | $92.22 Billion (2024 value) 1 | N/A | N/A | N/A | Dominant market share, robust infrastructure, high R&D investment. |
| Asia-Pacific Region | N/A | N/A | 2025-2030 | 10.76% (Biologics) 9 | Fastest-growing region, cost advantages, expanding local industry. |
Table 1: Global CDMO Market Forecast (2025-2034)
Section 2: The Selection Gauntlet: A Framework for Due Diligence and Partner Vetting
Choosing the right CDMO is arguably the most critical decision in the outsourcing lifecycle. A successful selection lays the foundation for a productive partnership, while a poor choice can lead to cascading failures, including timeline delays, budget overruns, and catastrophic quality issues. The process must be approached not as a simple procurement exercise but as a strategic risk management initiative. This requires a disciplined, multi-stage framework that begins with rigorous internal preparation and proceeds through a comprehensive, multi-domain external evaluation.
2.1 Defining Your Needs: The Internal Scorecard
Before any external engagement, a sponsor company must first achieve internal clarity and alignment. A common pitfall is to rush into the market with a vaguely defined project, which inevitably leads to scope creep, misaligned expectations, and an inability to conduct meaningful “apples-to-apples” comparisons between potential partners.23
The foundational step is to conduct a thorough internal assessment to precisely define the project’s scope and technical requirements.23 This process must be cross-functional, incorporating input from key stakeholders in Research & Development (R&D), Chemistry, Manufacturing, and Controls (CMC), Quality Assurance (QA), regulatory affairs, and supply chain management. This collaborative effort ensures that all facets of the project are considered, from scientific specifications to regulatory pathways and logistical needs.
The primary output of this internal phase should be a detailed Request for Proposal (RFP).5 The RFP serves as the primary communication tool with potential CDMOs and must translate the internal project requirements into objective, actionable benchmarks.26 For example, instead of a vague requirement for “biologics experience,” a well-defined criterion would specify a “minimum of five years of aseptic fill-finish experience with monoclonal antibodies and a track record of three or more commercial launches”.5
To guide the evaluation process, the internal team should develop a structured scorecard with weighted criteria.27 This tool forces a critical discussion about priorities, compelling the team to distinguish between “must-have” capabilities (e.g., cGMP compliance for sterile manufacturing) and “nice-to-have” attributes (e.g., geographic proximity).26 This structured approach transforms a potentially subjective decision into a more objective, data-driven analysis, providing a clear and defensible rationale for the final selection.
2.2 The Spectrum of Choice: One-Stop-Shop vs. Best-of-Breed
A fundamental strategic decision confronting every sponsor is the type of outsourcing model to pursue. The market generally offers two distinct approaches, each with a unique profile of benefits and risks.
The One-Stop-Shop (Integrated CDMO): This model involves partnering with a large, integrated CDMO that offers a comprehensive suite of services, potentially covering the entire lifecycle from process development and API manufacturing to finished drug product formulation, fill-finish, and packaging.12
- Pros: The primary advantage is simplified management. Having a single point of contact streamlines communication, reduces the administrative burden of managing multiple contracts and vendors, and can facilitate smoother and faster transitions between development stages.12
- Cons: The risks are significant. A smaller sponsor may become a low-priority client within a large CDMO’s vast portfolio, feeling like a “small fish in a big pond” and struggling to get the attention their project requires.21 These large organizations can also be hampered by bureaucracy, leading to slower decision-making and less flexibility.21 Furthermore, while they offer breadth, they may lack the profound depth of expertise in a highly specialized or novel technology that a boutique firm might possess. A telling case study revealed a company’s attempt to implement a one-stop-shop strategy failed because the high-volume sterile manufacturing specialists they evaluated did not bundle development services at a single site, forcing them into a hybrid, multi-vendor approach despite their initial intent.22
The Best-of-Breed (Specialized/Boutique CDMO): This model involves selecting multiple, smaller CDMOs, each chosen for its world-class expertise in a specific service or technology area.2
- Pros: This approach provides access to the highest level of specialized expertise for each stage of the project. Boutique CDMOs are often more flexible, agile, and innovative within their niche.21 They typically offer more personalized service, direct access to senior scientists, and more transparent communication, as the sponsor’s project represents a more significant portion of their business.21
- Cons: The primary drawback is complexity. The sponsor bears the full responsibility for managing multiple vendors, contracts, and relationships. The most significant risk lies in the technology transfers between different CDMOs, which are notoriously complex and can introduce delays and quality issues if not managed flawlessly.22 Additionally, a small, specialized firm may lack the capacity to scale up production for later clinical phases or commercial launch, potentially forcing a difficult and costly move to a larger partner mid-stream.21
The optimal choice is not universal; it depends entirely on the sponsor’s specific context. A virtual biotech with minimal internal project management resources might favor the simplicity of a one-stop-shop, accepting the associated risks. Conversely, a company with a highly novel and complex technology may determine that only a best-of-breed strategy can provide the necessary technical excellence. A case study involving a proprietary coating process illustrated this perfectly: the innovator company found that only a flexible, best-of-breed partner was willing to work with their unique, IP-protected process, whereas larger, more rigid CDMOs insisted on redeveloping the process to fit their standard equipment.22
2.3 The Due Diligence Deep Dive: A Multi-Domain Assessment
Once a shortlist of potential partners is identified, a rigorous and multi-faceted due diligence process is essential to vet their capabilities and mitigate the risk of a failed partnership.5 This investigation must go far beyond a review of marketing materials and facility tours.
- Technical and Technological Capabilities: The assessment must confirm that the CDMO has specific, demonstrable experience with the sponsor’s molecule type (e.g., small molecule, mAb, peptide), delivery form (e.g., oral solid, sterile injectable), and therapeutic area.6 It is critical to evaluate their equipment and facilities for suitability and, crucially, for their ability to scale production to meet future clinical and commercial needs.14 The CDMO’s approach and track record with technology transfer is a key indicator of their operational competence and should be scrutinized.23 An honest self-assessment of capabilities by the CDMO is a positive sign; a partner who claims they can do everything without acknowledging any limitations should be viewed with caution.32
- Quality and Regulatory Compliance: This is a non-negotiable area of diligence. The sponsor must conduct a thorough review of the CDMO’s Quality Management System (QMS), their history of cGMP compliance, and their experience with the specific regulatory authorities in the sponsor’s target markets (e.g., FDA, EMA).5 A history of successful agency inspections and a clean regulatory record are paramount.26 It is a best practice to conduct a formal quality audit
before a contract is signed to proactively identify any potential gaps or risks in their quality systems.5 - Operational and Project Management Excellence: A world-class facility is ineffective without a strong project management culture to support it.4 The due diligence team should evaluate the CDMO’s project management systems, their standard communication protocols, their change control processes, and their documented risk mitigation strategies.4 A crucial step is to request a meeting with the specific project manager and team lead who would be assigned to the program, as their individual experience and communication skills can make or break the collaboration.27
- Financial Stability and Business Health: A partnership is a long-term commitment, making the CDMO’s financial stability a critical factor.36 The assessment should review their financial health, their history of capital investment in facilities and technology, and their business continuity plans.29 Another key risk factor to investigate is client concentration. A CDMO that is overly reliant on a single large client could face instability if that client’s program is delayed or cancelled. A case study of a due diligence process conducted by a consulting firm for a CDMO acquisition uncovered exactly this risk, allowing the buyer to negotiate protective clauses into the deal.29
- Cultural Fit and Communication: Misaligned corporate cultures, communication styles, and working practices are a common source of friction and failure in CDMO partnerships.14 The evaluation should assess the CDMO’s transparency, responsiveness, and problem-solving approach.28 Direct conversations about working styles, meeting frequency, and escalation paths for issues should happen early.27 Geographic and cultural proximity can also be a significant advantage, streamlining collaboration by aligning time zones and business practices.32
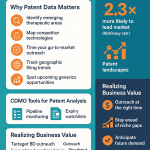
2.4 Leveraging Patent Intelligence for Strategic Vetting
A novel and powerful layer of due diligence involves the strategic use of patent intelligence. While traditional due diligence verifies a CDMO’s stated capabilities, analyzing their patent portfolio can reveal their demonstrated and emerging capabilities, providing an objective, data-driven view that cuts through marketing claims.
By analyzing a CDMO’s patent filings, a sponsor can gain several strategic advantages:
- Validate Technical Expertise: A CDMO that claims deep expertise in a specific technology, such as lipid nanoparticle (LNP) formulation or continuous manufacturing, should have a corresponding portfolio of patents in that area. A robust patent portfolio serves as documented, legally scrutinized proof of their inventive activity and genuine expertise, going far beyond a simple list of equipment on their website.38
- Identify True R&D Focus: Patent filing trends over time reveal a CDMO’s strategic direction. They show where the organization is consistently investing its R&D resources, which is a strong indicator of their future capabilities and areas of strategic importance.38 This can help a sponsor determine if a CDMO’s long-term strategy aligns with their own product pipeline.
- Enable Highly Targeted Partnering: Sponsors can use patent databases to search for solutions to their specific technical challenges. For instance, a company developing a highly potent API can identify CDMOs that are actively patenting novel containment or handling technologies. This allows for highly targeted outreach to partners who have already demonstrated an ability to solve the exact problems the sponsor is facing.38 A case study highlighted a CDMO that successfully used this strategy in reverse: by analyzing the patent landscape, they identified a growing need for LNP manufacturing, invested in the capability, and built a successful new service line to meet the emerging market demand.38
This use of patent intelligence adds an objective, evidence-based dimension to the selection process. It allows a sponsor to independently verify a CDMO’s technical prowess and strategic intent, providing a much deeper and more reliable assessment than can be achieved through conventional methods alone.
| Category | Specific Criterion | Weighting (1-5) | Score (1-10) | Weighted Score | Comments/Rationale |
| Technical Capability | Experience with specific molecule type (e.g., mAb, ADC, peptide) | 5 | |||
| Experience with required dosage form (e.g., sterile injectable, OSD) | 5 | ||||
| Proven track record in required scale of manufacturing (>2000L, etc.) | 4 | ||||
| Documented tech transfer process and success rate | 5 | ||||
| Analytical development and validation capabilities | 4 | ||||
| Quality & Regulatory | History of successful FDA/EMA inspections | 5 | |||
| Robustness of Quality Management System (QMS) | 5 | ||||
| Experience with regulatory submissions in target markets | 4 | ||||
| Data integrity systems and compliance (21 CFR Part 11) | 4 | ||||
| Operational & PM | Dedicated and experienced project management team | 5 | |||
| Clear communication protocols and escalation pathways | 4 | ||||
| Use of modern project management/collaboration tools | 3 | ||||
| Proactive risk management and contingency planning processes | 4 | ||||
| Business & Financial | Long-term financial stability and profitability | 4 | |||
| History of investment in technology and facilities | 3 | ||||
| Client concentration risk (not over-reliant on one client) | 3 | ||||
| Transparent and fair pricing structure | 4 | ||||
| Cultural Fit | Alignment of working styles and corporate culture | 3 | |||
| Demonstrated transparency and collaborative mindset | 4 | ||||
| Geographic and time-zone alignment | 2 | ||||
| Total | SUM |
Table 2: CDMO Selection Scorecard Template
Section 3: Architecting the Partnership: Legal and Contractual Frameworks
Once a preferred CDMO partner has been selected through rigorous due diligence, the relationship must be formalized through a set of meticulously crafted legal agreements. These documents—primarily the Master Service Agreement (MSA) and the Quality Agreement (QA)—are far more than legal formalities. They are the foundational architecture of the partnership, serving as strategic instruments that define roles, allocate risk, protect intellectual property (IP), and establish the rules of engagement for the entire collaboration. A failure to invest the necessary time and expertise in negotiating these contracts is a common and costly mistake.
3.1 The Master Service Agreement (MSA)
The MSA is the constitutional document for the business relationship. It establishes the overarching legal, financial, and operational framework that will govern all subsequent projects, which are typically detailed in individual Statements of Work (SOWs) or work orders. This structure provides efficiency, as it eliminates the need to renegotiate fundamental terms for each new project.40 A robust MSA should be designed with modularity in mind, creating a strong core agreement with clear mechanisms for adding project-specific details.41
Key clauses that require careful negotiation include:
- Scope of Services: This section must precisely define the categories of services to be provided by the CDMO. Crucially, it must also establish a clear and formal process for managing any changes in scope.40 This “change order” process is vital for preventing “scope creep,” where additional work is requested informally, leading to budget overruns and disputes about payment.41
- Payment Terms: The MSA must clearly detail the pricing model (e.g., fixed-fee per batch, time and materials), the invoicing schedule, payment deadlines, and any penalties for late payments.40 For international agreements, currency considerations must also be addressed.41
- Governance and Dispute Resolution: A well-defined governance structure, often including a Joint Steering Committee with representatives from both parties, should be outlined in the MSA. This clause should also specify the formal escalation procedures for unresolved issues and the agreed-upon mechanism for dispute resolution, such as mediation or arbitration, as an alternative to costly litigation.40
- Term and Termination: This clause provides clarity on the initial duration of the agreement, the conditions for renewal, and the specific circumstances under which either party can terminate the contract. It is essential to define conditions for both “termination for cause” (e.g., a material breach of the agreement) and “termination without cause” (which typically requires a longer notice period).41
- Indemnification and Limitation of Liability: These are often the most heavily negotiated clauses in the entire MSA because they directly allocate financial risk.5 The indemnification clause specifies when one party will cover the legal costs or damages of the other, typically for third-party claims. The limitation of liability clause sets a cap on the total financial damages one party can be held responsible for. CDMOs will almost always seek to cap their liability (often to the value of fees paid under the SOW) and exclude liability for consequential damages like the sponsor’s lost profits from a delayed launch.43 Sponsors, in turn, will negotiate for specific “carve-outs” to this cap for issues like breaches of confidentiality, IP infringement, or “bad acts” such as gross negligence or willful misconduct, which are directly within the CDMO’s control.43 A fair resolution for a batch failure often involves the CDMO waiving its fees for a re-run while the sponsor covers the cost of new raw materials.43
3.2 The Quality Agreement (QA)
The Quality Agreement is a distinct, legally binding document that is mandated by regulatory bodies like the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA).33 Its sole purpose is to define, in unambiguous detail, all roles and responsibilities related to Current Good Manufacturing Practices (cGMP) and product quality.33 It is the operational roadmap for ensuring the quality, safety, and efficacy of the manufactured product.
Essential elements of a comprehensive QA include:
- Delineation of Responsibilities: The agreement must explicitly state which party is responsible for every quality-related activity. This includes raw material testing and release, in-process testing, final product testing, analytical method validation, equipment qualification, batch record review, and, most critically, the final decision to release a batch.33
- Change Control and Deviations: The QA must detail the formal process for proposing, reviewing, approving, and documenting any change to the manufacturing process, equipment, materials, or analytical methods. It must also define the protocol for investigating, documenting, and resolving any deviation from an approved procedure, including the responsibilities for implementing Corrective and Preventive Actions (CAPA).33
- Audits and Inspections: The sponsor’s right to audit the CDMO’s facilities, quality systems, and documentation must be clearly codified. The QA should also outline the procedures for how the two parties will collaborate to manage and respond to inspections by regulatory authorities like the FDA.33
- Data Integrity: With the increasing reliance on electronic systems, the QA must establish clear expectations for electronic record-keeping, data security, audit trails, and compliance with specific regulations such as 21 CFR Part 11 in the U.S. and Annex 11 in the EU.33
- Supply Chain Management: The agreement should address the sponsor’s oversight of the CDMO’s supply chain. This includes the sponsor’s right to review the qualification of the CDMO’s raw material suppliers and ensure the traceability and integrity of all materials used in the product.33
For advanced therapies like cell and gene therapies, the QA takes on even greater importance. Given the “living” nature of these products, the agreement must incorporate robust governance policies for handling biological variability and a framework for collaboratively addressing the unexpected scientific and manufacturing challenges that frequently arise.44
3.3 Navigating Intellectual Property (IP): Protecting Your Core Assets
For many pharmaceutical and biotech companies, particularly emerging ones, their intellectual property is their most valuable asset. The IP clauses within the MSA are therefore of paramount strategic importance and are often the most contentious part of the negotiation. These clauses must meticulously address the ownership and use of both “background IP” (IP that each party brings into the collaboration) and “foreground IP” (IP that is newly created during the course of the project).5
The choice of an IP ownership model has profound and lasting implications for a sponsor’s future commercial strategy, its competitive landscape, and its ability to switch manufacturers if necessary.5 Several models exist, each with distinct advantages and disadvantages:
- Customer Owns Developed IP: In this model, the sponsor retains ownership of all foreground IP, often based on the principle that “I paid for it, I bought it”.5 This is generally the preferred model for sponsors as it provides maximum control over the asset and the freedom to move the project to another manufacturer without restriction.5
- CDMO Owns Developed IP: The CDMO may argue that any improvements to a manufacturing process developed at their facility using their equipment and personnel should belong to them. If the CDMO owns this new IP, they can prevent the sponsor from transferring that improved process to a competitor, creating a powerful form of “vendor lock-in”.5
- Joint Ownership: While this may seem equitable, it can be practically unworkable. Joint ownership often leads to complex disputes regarding who has the right to license the IP, who is responsible for defending it against infringement, and how any resulting revenue is shared.5
- Ownership Follows Inventorship: This model assigns ownership based on the legal standard of which party’s employees conceived the invention. This often favors the CDMO, as their scientists and engineers are the ones performing the work and are more likely to be named as inventors.5
A critical goal for the sponsor is to prevent “IP contamination,” a situation where the CDMO’s proprietary background IP becomes inextricably linked to the sponsor’s product or process.5 To safeguard against vendor lock-in and ensure supply chain continuity, sponsors should negotiate for, at a minimum, a broad, royalty-free, sublicensable license to all foreground IP. A further protective measure is a “springing license,” which grants the sponsor a license to the CDMO’s essential background IP that only becomes effective under specific trigger conditions, such as the CDMO’s failure to supply or insolvency.5 This provides a critical escape hatch, allowing the sponsor to transfer the complete process to another manufacturer in a crisis.
3.4 Freedom to Operate (FTO): Clarifying Responsibility
Freedom to Operate (FTO) is a legal analysis conducted to determine whether a proposed commercial product or process is at risk of infringing on the valid and enforceable patent rights of a third party.45 There is often a dangerous ambiguity in CDMO relationships about which party is responsible for FTO.
The ultimate responsibility for ensuring FTO for the product being sold rests squarely with the sponsor. It is the sponsor who is commercializing the product and who would be the primary target of an infringement lawsuit.46 A CDMO is a service provider and is not in a position to, nor will it agree to, provide a legal opinion on whether its client’s product infringes third-party patents.
However, the CDMO does have a responsibility regarding its own operations. The MSA should contain a clear warranty from the CDMO stating that its own internal manufacturing processes and platform technologies do not, to its knowledge, infringe on any third-party patents.42
The contract must make this division of responsibility explicit to avoid a catastrophic misunderstanding where each party assumes the other is handling FTO. The agreement should also define how the parties will cooperate and allocate costs if a third-party infringement claim arises that is related specifically to the manufacturing process used by the CDMO. This contractual clarity is essential for mitigating a significant and often overlooked legal risk.
| IP Ownership Model | Description | Sponsor Pros | Sponsor Cons | CDMO Pros | CDMO Cons |
| Customer Owns Developed IP | Sponsor retains full ownership of all IP created during the project. | Maximum control; full freedom to operate; easy to switch manufacturers; can block competitors. | May be charged a premium for the work; may still require a license to CDMO’s background IP. | Simple, clean transaction. | No long-term IP asset; cannot leverage improvements for other clients. |
| CDMO Owns Developed IP | CDMO retains ownership of process improvements and other created IP. | May result in lower initial project costs. | Creates “vendor lock-in”; difficult to switch manufacturers; reliant on CDMO’s IP strategy. | Builds valuable IP portfolio; creates sticky client relationships; can leverage improvements across client base. | May need to grant broad, royalty-free licenses, limiting direct revenue from the IP.5 |
| Joint Ownership | Both parties co-own the developed IP. | Appears equitable and can avoid difficult upfront negotiations. | Practically unworkable; leads to complex disputes over control, licensing, and enforcement. | Shares in potential upside of valuable IP. | Shares control; may be unable to use IP without sponsor’s consent, limiting its value. |
| Ownership Follows Inventorship | Ownership is assigned based on the legal definition of inventorship. | Follows a clear legal standard. | Often favors the CDMO, as their employees are typically the inventors; can lead to vendor lock-in. | Often results in ownership of process improvements. | Ownership can be fragmented and uncertain until inventorship is formally determined. |
| Springing License to Background IP | Sponsor is granted a license to CDMO’s essential background IP, effective only upon a trigger event (e.g., failure to supply). | Critical risk mitigation tool; ensures supply chain continuity and ability to tech transfer in a crisis. | May be difficult to negotiate; scope of license needs to be precisely defined. | Can be used as a negotiating tool to offer assurance to the sponsor. | Grants a third party rights to core proprietary technology, even if contingent. |
Table 3: Comparative Analysis of IP Ownership Models
Section 4: Executing the Collaboration: Operational Management and Integration
With robust contracts in place, the focus shifts from planning to execution. A successful CDMO collaboration is not self-sustaining; it requires continuous, proactive management. The day-to-day reality of making the partnership work rests on three operational pillars: a well-structured joint project management and governance system, a meticulously planned and executed technology transfer, and a seamlessly integrated supply chain. Failure in any of these areas can undermine even the best-laid plans and contractual agreements.
4.1 Joint Project Management and Governance
Effective governance transforms a relationship from a series of transactional exchanges into a strategic partnership. It provides the structure for oversight, communication, and decision-making that is essential for navigating the complexities of drug development.4
A best-practice governance framework is typically multi-tiered:
- Joint Steering Committee (JSC): This is a high-level body composed of senior leaders from both the sponsor and the CDMO. The JSC provides strategic oversight, is responsible for resolving major issues that cannot be handled at the operational level, and ensures the partnership remains aligned with the sponsor’s corporate goals. It typically meets on a monthly or quarterly basis.4
- Operational Working Groups: These are the functional teams responsible for the day-to-day execution of the project. They consist of scientists, engineers, and project managers from both organizations who collaborate on specific tasks. These teams require frequent interaction, often through weekly status calls and daily check-ins during critical phases.4
Communication is the lifeblood of this structure. A formal communication plan is critical and should be established at the outset.24 This plan should define the frequency and format of regular meetings, establish clear escalation pathways for problems, and designate responsible points of contact at each level.4 “Overcommunication” is consistently cited as a key success factor, ensuring that both parties have a clear and current understanding of project status and can troubleshoot issues proactively.28 The use of modern collaborative tools, such as shared project management software (e.g., MS Project), and secure data-sharing platforms (e.g., SharePoint, MS Teams), is essential for maintaining real-time alignment and transparency.24
Finally, the partnership must be managed by data. The joint team should define and regularly track a set of Key Performance Indicators (KPIs) that objectively measure performance against agreed-upon goals. These KPIs typically include metrics such as batch success rates, deviation rates, adherence to timelines, and “on-time, in-full” delivery.4 This data-driven approach creates mutual accountability and provides a factual basis for performance reviews and continuous improvement efforts.
4.2 The Technology Transfer Playbook
The technology transfer (tech transfer)—the process of moving a manufacturing process from a sending unit to a receiving unit—is a notoriously high-risk and complex phase of any outsourcing project.19 A successful transfer requires treating it as a formal project in its own right, with a dedicated plan, a dedicated team, and a disciplined, stage-gated process.31 The common pitfall is to treat it as a simple documentation hand-off, which often leads to costly failures.
The first step is to assemble a dedicated, multidisciplinary tech transfer team. This team must include subject matter experts from all relevant functions—process development, manufacturing, quality assurance, quality control, and regulatory affairs—from both the sending and receiving organizations.31 It is crucial that team members are introduced to their counterparts early in the process to build rapport and establish effective lines of communication.31
The tech transfer process itself should follow a structured playbook:
- Gap Analysis and Feasibility Assessment: Before any physical transfer begins, the team must conduct a thorough paper-based gap analysis. This involves meticulously comparing the requirements of the manufacturing process with the capabilities, equipment, and procedures of the receiving CDMO site.31 This analysis is designed to proactively identify any potential gaps in equipment compatibility, scale, or compliance with cGMP requirements.31
- Comprehensive Documentation: The sending unit must provide a complete and detailed tech transfer package. This is more than just a process description; it must include detailed Standard Operating Procedures (SOPs), a full history of process development, specifications for all raw materials, and validated analytical methods.24
- Joint Risk Assessment: The team should collaboratively perform a risk assessment to identify the Critical Quality Attributes (CQAs) of the product and the Critical Process Parameters (CPPs) of the manufacturing process. This ensures that the process controls at the new site are robust enough to consistently produce a product that meets all quality specifications.31
- On-Site Execution and Verification: The transfer culminates in physical execution at the CDMO’s facility. It is highly recommended to perform one or more non-GMP engineering or feasibility runs before committing to a full GMP batch.24 While these runs add time and cost, they are invaluable for de-risking the process, training the new operating team, and demonstrating that the process is reproducible at the new site. A widely adopted best practice is the
“person-in-plant” approach, where key personnel from the sending unit are physically present at the CDMO facility during these runs. This allows for real-time observation, guidance, and collaborative troubleshooting, which is far more effective than remote communication.31
4.3 Integrating the Supply Chain
In today’s volatile global environment, supply chain management has evolved from a back-office logistical function into a strategic imperative that is central to the success of a CDMO partnership.48 A resilient and transparent supply chain is a key value proposition of a top-tier CDMO.
Sponsors must evaluate a CDMO’s supply chain capabilities as part of their due diligence. Leading CDMOs are actively investing in making their supply chains more resilient to disruption. Key strategies include:
- Supplier Diversification: Reducing reliance on single-source suppliers for critical raw materials to mitigate the risk of shortages.49
- Strategic Sourcing and Geographic Diversification: Establishing procurement operations in multiple key regions (e.g., Europe, Asia, North America) to navigate geopolitical tensions and trade restrictions.49
- Rigorous Supplier Management: Implementing a formal, three-stage process for managing suppliers that includes selection, qualification, and ongoing evaluation and audits to ensure consistent quality and reliability.51
The most significant recent development in this area is the move toward digital integration and transparency. Modern CDMOs are implementing integrated data platforms that provide their clients with near real-time visibility into the entire manufacturing and supply chain process.49 These platforms can connect data from Enterprise Resource Planning (ERP), quality, laboratory, and process monitoring systems into a single, centralized dashboard. This allows the sponsor to remotely and securely access data, see the real-time status of their program, and monitor key metrics.49 This level of transparency builds trust, accelerates decision-making, and reduces timelines by eliminating the errors and delays associated with manual data transfer and reporting. This capability is transforming the CDMO supply chain from a potential source of risk into a shared strategic asset.
Section 5: Navigating Turbulence: Risk Management and Conflict Resolution
No complex, long-term partnership is without its challenges. In the high-stakes world of pharmaceutical development, where timelines are tight and scientific uncertainty is a given, issues will inevitably arise. The true measure of a CDMO partnership is not whether it encounters problems, but how it prepares for, manages, and resolves them. A proactive, structured approach to risk management and conflict resolution is essential for navigating turbulence and ensuring the collaboration remains productive and on track.
5.1 A Proactive Risk Management Framework
The most effective risk management is proactive, not reactive. The guiding principle for a sponsor’s oversight should be a “trust but verify” approach.4 This model balances the need to empower the CDMO with the autonomy to execute effectively while maintaining the necessary level of oversight to ensure quality and compliance. Micromanaging a CDMO can stifle innovation and slow progress, while a completely hands-off approach exposes the sponsor to unacceptable risks.4
A proactive framework begins with identifying the most common and high-impact risks inherent in CDMO partnerships:
- Timeline Delays: These are a frequent challenge, often caused by the CDMO having unforeseen capacity constraints, difficulties during technology transfer, raw material shortages, or simply poor project planning.6
- Quality and Regulatory Issues: A CDMO with an inadequate Quality Management System (QMS), poor data integrity practices, or a failure to adhere strictly to cGMP standards can lead to devastating consequences. These include out-of-specification results, batch failures, product recalls, and severe regulatory actions such as an FDA warning letter.6
- Supply Chain Disruptions: The inability to secure critical raw materials, single-use components, or other supplies can bring production to a complete halt, jeopardizing clinical trial timelines and commercial supply.9
- Scope Creep and Cost Overruns: If the project scope is not meticulously defined and managed, informal requests and unforeseen technical challenges can lead to significant, unbudgeted costs, particularly in a time-and-materials contract model.24
Once identified, these risks must be actively managed through a combination of strategies:
- Rigorous Upfront Due Diligence: The single most effective risk mitigation strategy is to select the right partner from the outset. A thorough due diligence process that vets a CDMO’s technical, quality, and operational capabilities is the best defense against future problems.37
- Robust Contractual Protections: The MSA and QA should be used as strategic tools to clearly allocate risk and define responsibilities for quality and performance.5
- Comprehensive Contingency Planning: Prudent project management involves building buffer time into project timelines to absorb unexpected delays. It is also a best practice to set aside a contingency budget, typically 10-15% of the total project cost, to cover unexpected expenses or scope changes.24 As one expert advises, it is critical to always have a “Plan C”.53
- Regular Audits and Performance Reviews: The “verify” component of the model involves conducting periodic on-site or virtual audits to review documentation, observe processes, and verify compliance with the Quality Agreement. These audits are not merely policing actions; they are opportunities to strengthen collaboration and identify areas for improvement.4
5.2 The High Cost of Failure: Quantifying the Impact
A failed CDMO partnership can have severe and cascading consequences that extend far beyond the immediate project.6 Understanding the full scope of this impact is critical for appreciating the importance of proactive risk management.
- Financial Impact: The direct financial costs are often the most visible. This includes the value of lost API and other raw materials in a failed batch, the fees paid to the CDMO for the work, and the significant expense of troubleshooting the failure and re-running the manufacturing campaign. In one real-world case, a single IT failure at a CDMO resulted in a direct loss of USD 1 million.55 These costs are magnified by the enormous overall investment in drug development, where the average cost to bring a single product to market can exceed USD 2.6 billion.5
- Strategic Impact: The most devastating cost of a CDMO failure is often the irreversible loss of time. A significant delay in the manufacturing of clinical trial materials can push back a pivotal study, and a delay in commercial production means a delay in market entry.5 In the pharmaceutical industry, where products have a finite period of patent-protected market exclusivity, this is a catastrophic strategic loss. Every day of delay is a day of lost revenue at peak, monopoly prices, a cost that can easily run into the millions of dollars and which permanently erodes the product’s lifetime value and competitive standing.5
- Reputational and Regulatory Impact: Quality failures can have severe consequences that are difficult to quantify. A product recall can cause irreparable damage to a company’s reputation with patients, physicians, and investors.6 A serious regulatory finding, such as an FDA warning letter issued to a CDMO partner, creates a crisis for the sponsor. It triggers tremendous anxiety and necessitates an immediate and resource-intensive investigation to determine the impact on product quality and patient safety, potentially leading to a recall and a complete halt of manufacturing at the site.52
5.3 Conflict Resolution Mechanisms
Conflict is an inevitable part of any complex, high-stakes collaboration. The key to a durable partnership is not the absence of conflict, but the presence of a structured and constructive framework for resolving it.57
The best approach to conflict is prevention. A meticulously detailed project plan that is clearly aligned with the sponsor’s initial RFP is the most powerful tool for preventing misunderstandings.57 This, combined with a culture of frequent, transparent communication, can foster the trust needed to address small issues before they escalate into major disputes.24
When conflicts do arise, the following principles can guide a constructive resolution process:
- Utilize Predefined Escalation Pathways: The governance structure established in the MSA should be the first line of defense. When a problem cannot be resolved by the operational working group, it should be formally escalated to the Joint Steering Committee for a higher-level review and decision.4 This provides a clear, orderly process and prevents issues from festering.
- Seek to Understand Motivations: It is human nature to resort to finger-pointing during a conflict. A more effective approach is to step back and try to understand the other party’s motivations.57 Are they facing internal financial pressures? Are they constrained by an unreasonable timeline? Are they dealing with issues from another client? Often, a candid, heart-to-heart discussion can uncover the root cause of the problem, allowing the parties to work collaboratively on a solution that addresses both of their needs.57
- Practice Patience and Flexibility: Most significant conflicts are not resolved in a single meeting or with one well-worded email. Resolution is often an iterative process that requires patience and a willingness to be flexible in one’s approach.57 If one strategy is not working, it is important to assess why and adjust the approach rather than becoming locked into a single, ineffective tactic.
- Focus on the Problem, Not the People: It is crucial to manage the emotional component of conflict. Behaviors that can exacerbate a situation include a sense of entitlement on the sponsor’s part (e.g., “the CDMO must agree to every demand”) or dismissiveness on the CDMO’s part (e.g., “none of our other clients do it that way”).57 The focus must remain on a collaborative effort to fix the problem, get the project back on track, and prevent the issue from recurring in the future.59
| Risk Category | Specific Risk Example | Likelihood (H/M/L) | Impact (H/M/L) | Proactive Mitigation Strategy | Contingency Plan (If Risk Occurs) |
| Quality/Regulatory | GMP batch failure due to contamination or out-of-specification (OOS) result. | M | H | Rigorous due diligence of CDMO’s QMS and sterile practices; robust Quality Agreement; sponsor person-in-plant for critical steps. | Initiate formal deviation and CAPA investigation; use pre-qualified backup API/drug substance lot for re-run; assess impact on clinical/commercial timeline. |
| Timeline/Capacity | CDMO announces a 3-month delay due to scheduling conflicts with another client’s project. | M | H | During selection, assess CDMO’s capacity forecasting and project load; build buffer time into overall project plan; negotiate priority clauses in MSA. | Immediately escalate to JSC to assess impact; explore expediting with CDMO at a premium; initiate contact with pre-identified backup CDMO. |
| Supply Chain | A critical, single-source raw material becomes unavailable due to a supplier issue. | L | H | During development, identify high-risk materials; work with CDMO to qualify a second supplier for all critical materials; build safety stock. | Immediately activate qualified second supplier; draw down safety stock to cover production during transition; assess long-term viability of original supplier. |
| Technology Transfer | Tech transfer fails, resulting in a process that is not reproducible at the CDMO site. | M | H | Conduct thorough paper-based gap analysis; perform non-GMP engineering runs before GMP batch; utilize “person-in-plant” for on-site expertise. | Halt GMP production; form a joint troubleshooting team to diagnose the root cause; perform additional engineering runs to confirm process robustness before re-attempting GMP. |
| Cost/Budget | Project requires significant unplanned work, leading to “scope creep” and budget overruns. | H | M | Meticulously define project scope in the SOW; establish a formal, rigorous change order process in the MSA. | Allocate a 10-15% contingency budget at project outset; require all scope changes to go through formal change order process for cost and timeline impact assessment. |
Table 4: Risk Mitigation and Contingency Planning Matrix
Section 6: The Future of CDMO Partnerships: Strategic Recommendations and Outlook
The landscape of pharmaceutical outsourcing is in a state of dynamic evolution. The relationships between sponsors and CDMOs are maturing beyond simple, transactional arrangements into complex, deeply integrated strategic partnerships. To succeed in this new era, executive leaders must adopt a forward-looking perspective, embracing the trends that are reshaping the industry and implementing the strategic best practices that will define the next generation of successful collaborations.
6.1 The Evolving Partnership Model: From Transactional to Transformational
The traditional fee-for-service model, while still prevalent, is increasingly being supplemented by more sophisticated partnership structures designed to foster a shared vision and joint accountability.60 The goal is to move from a relationship where a sponsor simply pays for a service to one where both parties are mutually invested in the successful outcome of the project.
A key element of this evolution is the shift toward aligned incentives. Mature partnerships are exploring outcome-based pricing models that move away from traditional time- or volume-based contracts.60 For example, a contract might include milestone payments tied to successful batch completion or regulatory filing, or even a value-sharing component. While these models require more complex performance metrics and monitoring systems, they powerfully align the CDMO’s financial incentives with the sponsor’s primary goals of achieving speed, efficiency, and quality.60
Technology is the critical enabler of this transformational shift. The establishment of joint data governance and integrated digital platforms is foundational to building the level of transparency and trust required for a true strategic partnership.60 When a sponsor has secure, compliant, real-time access to their project’s manufacturing and quality data, it fundamentally changes the dynamic of the relationship. It allows for collaborative, data-driven decision-making and proactive problem-solving, rather than reactive responses to periodic reports.49 This digital integration is no longer a “nice-to-have” feature but a core component of a modern, high-functioning CDMO collaboration.
6.2 Key Takeaways and Actionable Recommendations for Executive Leadership
Synthesizing the analysis of the market, selection processes, legal frameworks, and operational realities, several key strategic recommendations emerge for executive leadership seeking to maximize the value and minimize the risk of their CDMO partnerships.
- Elevate CDMO Management to a Core Strategic Function: The era of viewing outsourcing as a simple procurement task is over. Leadership must recognize that the ability to select, contract with, and manage CDMO partners is a core competency that directly impacts the company’s bottom line and competitive position. This requires a strategic investment in building a best-in-class internal procurement and contracting function staffed with skilled negotiators and relationship managers. Crucially, there must be seamless collaboration and alignment between this function and the business and scientific units to ensure that outsourcing strategies are always informed by overarching corporate goals.60
- Invest Holistically in the Partnership: A successful partnership is an asset that requires continuous investment of time, resources, and human capital. This goes beyond simply paying invoices. It means fostering strong, multi-level relationships between the sponsor and CDMO teams, from the scientists in the lab to the executives in the C-suite. It requires a commitment from leadership to champion a culture of transparency, open communication, and collaborative, blame-free problem-solving.59 This investment in the “soft” infrastructure of the relationship is what provides the resilience to withstand the inevitable pressures of drug development.
- Adopt a Proactive, Lifecycle-Oriented Governance Model: The partnership does not end when the MSA is signed. Effective collaboration requires continuous, proactive management across the entire drug development lifecycle. Executive leadership must sponsor and enforce a robust governance framework that includes regular performance reviews against clear KPIs, ongoing risk assessment and mitigation planning, and a shared commitment to quality and continuous improvement. This ensures that the partnership remains aligned and productive as the project evolves from early-phase development through to commercialization.
- Demand and Leverage Digital Integration: In the current technological landscape, executive leaders should establish digital transparency and integrated data platforms as a non-negotiable requirement when selecting and managing CDMO partners. The ability to have real-time, secure access to manufacturing, supply chain, and quality data is a powerful risk management tool and a significant driver of efficiency.49 Leaders should empower their teams to use this data to accelerate decision-making and hold partners accountable for performance.
- Integrate Geopolitical Reality into Supply Chain Strategy: The CDMO landscape is no longer shaped by market and scientific forces alone. The rise of economic nationalism and events like the BIOSECURE Act demonstrate that geopolitical risk is now a tangible and significant threat to supply chain continuity.3 Leaders must ensure that this new reality is integrated into their strategic planning. This means prioritizing supply chain resilience and diversification, carefully evaluating the geographic footprint of potential partners, and potentially shifting from a “just-in-time” to a “just-in-case” inventory strategy to navigate an increasingly uncertain world.
By embracing these strategic imperatives, pharmaceutical and biotechnology companies can move beyond the traditional, often fraught, client-vendor relationship. They can forge durable, transformational partnerships with their CDMOs that not only mitigate risk but also create profound, sustainable value—accelerating the delivery of life-changing medicines to patients worldwide.
Works cited
- CDMO Market Size, Share & Trends | Growth Analysis [2032] – Fortune Business Insights, accessed July 30, 2025, https://www.fortunebusinessinsights.com/contract-development-and-manufacturing-organization-cdmo-outsourcing-market-102502
- Biologics CDMO Market to Grow by USD 16.32 Billion (2025-2029 …, accessed July 30, 2025, https://www.prnewswire.com/news-releases/biologics-cdmo-market-to-grow-by-usd-16-32-billion-2025-2029-driven-by-cost-efficient-resources-in-emerging-markets-ai-impact-on-trends—technavio-302366945.html
- CDMOs: Challenges & Opportunities in a Big Year of Change | Contract Pharma, accessed July 30, 2025, https://www.contractpharma.com/cdmos-challenges-opportunities-in-a-big-year-of-change/
- How To Manage CDMOs: Best Practices For Development And Manufacturing – Outsourced Pharma, accessed July 30, 2025, https://www.outsourcedpharma.com/topic/managing-cdmo-relationships
- What to Look for in a CDMO Contract – DrugPatentWatch – Transform Data into Market Domination, accessed July 30, 2025, https://www.drugpatentwatch.com/blog/what-to-look-for-in-a-cdmo-contract/
- The five key risks of failing to select the right CDMO partner – Alcami, accessed July 30, 2025, https://www.alcami.com/blog/the-five-key-risks-of-failing-to-select-the-right-cdmo-partner/
- Five Trends That Will Redefine the CDMO Industry in 2025 – The Pharma Navigator, accessed July 30, 2025, https://www.thepharmanavigator.com/news/five-trends-that-will-redefine-the-cdmo-industry-in-2025
- Pharmaceutical Contract Development, Manufacturing, and Services: A Market Poised for Unprecedented Growth, accessed July 30, 2025, https://www.americanpharmaceuticalreview.com/Featured-Articles/618345-Pharmaceutical-Contract-Development-Manufacturing-and-Services-A-Market-Poised-for-Unprecedented-Growth/
- Biologics CDMO Market Size, Growth & Industry Forecast, 2025-2030, accessed July 30, 2025, https://www.mordorintelligence.com/industry-reports/biologics-contract-development-and-manufacturing-organization-cdmo-market
- Investigational New Drug CDMO Market Size to Hit USD 10.26 Bn …, accessed July 30, 2025, https://www.precedenceresearch.com/investigational-new-drug-cdmo-market
- Pharmaceutical Contract Manufacturing Market Report, 2030 – Grand View Research, accessed July 30, 2025, https://www.grandviewresearch.com/industry-analysis/pharmaceutical-contract-manufacturing-market-report
- The CDMO Surge: Why Pharma Is Outsourcing More Than Ever – News-Medical.net, accessed July 30, 2025, https://www.news-medical.net/life-sciences/The-CDMO-Surge-Why-Pharma-Is-Outsourcing-More-Than-Ever.aspx
- What Are The Benefits of Partnering With a CDMO? – Bachem, accessed July 30, 2025, https://www.bachem.com/articles/commercial-apis/what-are-the-benefits-of-partnering-with-a-cdmo/
- CDMO Outsourcing Explained: Benefits and Insights – Upperton Pharma Solutions, accessed July 30, 2025, https://upperton.com/navigating-cdmo-outsourcing-a-practical-guide/
- How CDMOs are leading innovation for pharmaceutical partners – EY, accessed July 30, 2025, https://www.ey.com/en_gl/insights/strategy/how-cdmo-companies-are-leading-innovation-for-pharmaceutical-partners
- Investigational New Drug CDMO Market Companies: Size, Trends, and Key Players, accessed July 30, 2025, https://www.healthcarewebwire.com/investigational-new-drug-cdmo-market-companies/
- Optimizing Outsourcing: A Case Study on Evaluating CDMO Capabilities for API and FD Development – PR Newswire, accessed July 30, 2025, https://www.prnewswire.com/news-releases/optimizing-outsourcing-a-case-study-on-evaluating-cdmo-capabilities-for-api-and-fd-development-302341149.html
- Top 6 Companies in the Global Contract Development and Manufacturing Organisation (CDMO) Market in 2025, accessed July 30, 2025, https://www.expertmarketresearch.com/healthcare-articles/top-cdmo-companies
- The Pros & Cons of Internal Facilities and CDMOs for Biopharmaceutical Companies | APC, accessed July 30, 2025, https://blog.combination.com/the-pros-cons-of-internal-facilities-and-cdmos-for-biopharmaceutical-companies/
- CDMO industry projects growth, spurns BIOSECURE: CPHI research – Fierce Pharma, accessed July 30, 2025, https://www.fiercepharma.com/pharma/cdmo-growth-could-accelerate-2025-industry-spurns-biosecure-cphi-milan
- The Advantages Of A Boutique CDMO Vs. Challenges Of A Large …, accessed July 30, 2025, https://lgmpharma.com/blog/the-advantages-of-a-boutique-cdmo-vs-challenges-of-a-large-cdmo-how-to-choose-the-right-partner/
- One-Stop Shop Or Best of Breed 3 CDMO Selection Case Studies – Outsourced Pharma, accessed July 30, 2025, https://www.outsourcedpharma.com/doc/one-stop-shop-or-best-of-breed-cdmo-selection-case-studies-0001
- Best Practices for CDMO Evaluation, Selection, and Management – Precision For Medicine, accessed July 30, 2025, https://www.precisionformedicine.com/blog/best-practices-for-cdmo-evaluation-selection-and-management/
- 6 Common Pitfalls in CDMO Partnerships—and How to Avoid Them – List Labs, accessed July 30, 2025, https://listlabs.com/6-common-pitfalls-in-cdmo-partnerships-and-how-to-avoid-them/
- ASHP Guidelines on Outsourcing Pharmaceutical Services, accessed July 30, 2025, https://www.ashp.org/-/media/assets/policy-guidelines/docs/guidelines/outsourcing-pharmaceutical-services.pdf
- How To Select The Right CDMO – Outsourced Pharma, accessed July 30, 2025, https://www.outsourcedpharma.com/topic/identifying-selecting-cdmo
- Introduction to CDMO partnerships – 3Biotech, accessed July 30, 2025, https://www.3biotech.com/post/introduction-to-cdmo-partnerships
- What is a CDMO and what to look for in a partner – Patheon pharma …, accessed July 30, 2025, https://www.patheon.com/us/en/insights-resources/blog/what-is-a-cdmo.html
- Uncovering Hidden Risks and Value – Quality Executive Partners, accessed July 30, 2025, https://www.qualityexecutivepartners.com/thought-leadership/uncovering-hidden-risks-and-value
- Choosing A CDMO: Key Considerations – PCI Services, accessed July 30, 2025, https://pci.com/resources/choosing-a-cdmo-key-considerations/
- Key considerations for biologics CDMO tech transfer – GTP Bioways, accessed July 30, 2025, https://www.gtp-bioways.com/tips-tricks/key-considerations-biologics-cdmo-tech-transfer/
- Maximizing Value from Your CDMO Partnership: Best Practices and Insights – GL CHEMTEC, accessed July 30, 2025, https://blog.glchemtec.ca/blog/maximizing-value-from-cdmo-partnerships
- The Importance Of Quality Agreements In CDMO Partnerships – Compliance Architects, accessed July 30, 2025, https://compliancearchitects.com/cdmo-partnerships/
- Top 10 Factors to Consider When Choosing a CDMO, accessed July 30, 2025, https://renejix.com/criteria-for-cdmo-selection/
- CDMO Project Management | Bachem, accessed July 30, 2025, https://www.bachem.com/products/services-and-capabilities/project-management/
- Top 5 Most Important Compliance Challenges In CDMO Relationships, accessed July 30, 2025, https://compliancearchitects.com/cdmo-relationships/
- What Happens When Your CDMO Partnership Goes Wrong? – Patrick Wareing, accessed July 30, 2025, https://patrickwareing.com/news/what-happens-when-your-cdmo-partnership-goes-wrong/
- How CDMOs Can Use Patent Data to Win More Pharmaceutical Clients – DrugPatentWatch, accessed July 30, 2025, https://www.drugpatentwatch.com/blog/how-cdmos-can-use-patent-data-to-win-more-pharmaceutical-clients/
- Role of IP in competitive Intelligence gathering and analysis – WIPO, accessed July 30, 2025, https://www.wipo.int/edocs/mdocs/sme/en/wipo_ip_del_10/wipo_ip_del_10_theme05_4.ppt
- Mastering the Master Service Agreement (MSA): The Ultimate Guide – Contract Management Software | Concord, accessed July 30, 2025, https://www.concord.app/guide/master-service-agreement
- Master Service Agreement Guide 2025 (MSA) – ContractPodAi, accessed July 30, 2025, https://contractpodai.com/news/master-service-agreement-guide/
- Make The Service Agreement Work For You And Your CDMO – Outsourced Pharma, accessed July 30, 2025, https://www.outsourcedpharma.com/doc/make-the-service-agreement-work-for-you-and-your-cdmo-0001
- Negotiating a “Win-Win” in Pharmaceutical Contract Manufacturing | Outside GC, accessed July 30, 2025, https://www.outsidegc.com/blog/negotiating-a-win-win-in-pharmaceutical-contract-manufacturing
- Contract Manufacturing Quality Agreement: Defining Governance in …, accessed July 30, 2025, https://xtalks.com/webinars/contract-manufacturing-quality-agreement-defining-governance-in-cdmo-partnerships/
- How to Conduct a Freedom-to-Operate Analysis for a Drug …, accessed July 30, 2025, https://drugrepocentral.scienceopen.com/hosted-document?doi=10.58647/DRUGREPO.24.1.0011
- Freedom to Operate Opinions | Finnegan | Leading IP+ Law Firm, accessed July 30, 2025, https://www.finnegan.com/en/work/practices/diligence-licensing-and-opinions/freedom-to-operate-opinions.html
- Freedom to Operate Definition, Importance & Next Steps – Dilworth IP, accessed July 30, 2025, https://www.dilworthip.com/resources/news/freedom-to-operate/
- Building Better CDMO Supply Chain Readiness – Cryoport Systems, accessed July 30, 2025, https://www.cryoport.com/bio-blog-building-better-cdmo-supply-chain-readiness/
- CDMOs Are Making Their Supply Chains More Resilient & Secure, accessed July 30, 2025, https://resilience.com/blog/special-feature-outsourcing-formulation-development-manufacturing
- The Challenges CDMOs Face in 2025 – And How to Tackle Them – Mint CRM, accessed July 30, 2025, https://www.mintcrm.co.uk/articles/detail/cdmo-challenges-2025/
- CDMO Supply Chain Management – AGC Pharma Chemicals, accessed July 30, 2025, https://www.agcpharmachemicals.com/en/cdmo-services/services-capabilities/cdmo-supply-chain-management/
- What To Do When Your CDMO Receives A Warning Letter – Outsourced Pharma, accessed July 30, 2025, https://www.outsourcedpharma.com/doc/what-to-do-when-your-cdmo-receives-a-warning-letter-0001
- Navigating the CDMO-Sponsor Partnership | PDA – Parenteral Drug Association, accessed July 30, 2025, https://www.pda.org/pda-letter-portal/home/full-article/navigating-the-cdmo-sponsor-partnership
- What Happens When Your CDMO Partnership Goes Wrong? – The Pharma Navigator, accessed July 30, 2025, https://www.thepharmanavigator.com/news/what-happens-when-your-cdmo-partnership-goes-wrong
- Why pharmaceutical manufacturers can’t afford IT failures: A real-world CDMO case study, accessed July 30, 2025, https://blogs.manageengine.com/corporate/general/2025/01/13/why-pharmaceutical-manufacturing-cant-afford-it-failures-a-real-world-cdmo-case-study.html
- Counting the cost of failure in drug development – Pharmaceutical Technology, accessed July 30, 2025, https://www.pharmaceutical-technology.com/features/featurecounting-the-cost-of-failure-in-drug-development-5813046/
- When Conflict Arises With Your CDMO – Outsourced Pharma, accessed July 30, 2025, https://www.outsourcedpharma.com/doc/when-conflict-arises-with-your-cdmo-0001
- Managing Conflict: A Guide for the Pharmacy Manager – PMC – PubMed Central, accessed July 30, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC4568116/
- The Perils and Promise of Strategic Partnering with CROs | Pharmaceutical Outsourcing, accessed July 30, 2025, https://www.pharmoutsourcing.com/Featured-Articles/184119-The-Perils-and-Promise-of-Strategic-Partnering-with-CROs/
- Building a shared vision for pharma R&D–supplier partnerships – McKinsey, accessed July 30, 2025, https://www.mckinsey.com/industries/life-sciences/our-insights/building-a-shared-vision-for-pharma-r-and-d-supplier-partnerships
- McKinsey report highlights shift toward outsourcing pharma R&D, accessed July 30, 2025, https://www.grip.globalrelay.com/mckinsey-report-highlights-shift-toward-outsourcing-pharma-rd/
- Pharma’s Simplification Imperative: From McKinsey’s Vision to Implementation Reality, accessed July 30, 2025, https://medium.com/@laurence.grisel/pharmas-simplification-imperative-from-mckinsey-s-vision-to-implementation-reality-c8bfe20a4a19