Triamcinolone acetonide - Generic Drug Details
✉ Email this page to a colleague
What are the generic drug sources for triamcinolone acetonide and what is the scope of patent protection?
Triamcinolone acetonide
is the generic ingredient in twenty-seven branded drugs marketed by Abbvie, Chattem Sanofi, Astellas, Ivax Pharms, Delcor Asset Corp, Teva, Solvay, Actavis Mid Atlantic, Alkem Labs Ltd, Alpharma Us Pharms, Ambix, Chartwell Rx, Cosette, Encube, Fougera Pharms, Glenmark Pharms Ltd, Macleods Pharms Ltd, Micro Labs, Morton Grove, Mylan, Padagis Us, Pharmaderm, Pharmafair, Strides Pharma, Taro, Topiderm, Crown Labs, Savage Labs, Pacira Pharms Inc, Apothecon, Amneal, Eugia Pharma, Long Grove Pharms, Mylan Labs Ltd, Parnell, Sandoz, Teva Pharms Usa, Watson Labs, Allergan, Harrow Eye, Epic Pharma Llc, Pai Holdings Pharm, Quagen, Wockhardt Bio Ag, Aurobindo Pharma Ltd, Cintex Svcs, Glenmark Pharms, Padagis Israel, Cmp Pharma Inc, Akorn, Lyne, Lupin Atlantis, Sanofi Aventis Us, Apotex, Perrigo Pharma Intl, Sun Pharm Inds Inc, Rising, Saptalis Pharms, and Bausch And Lomb Inc, and is included in one hundred and thirty-nine NDAs. There are seven patents protecting this compound. Additional information is available in the individual branded drug profile pages.Triamcinolone acetonide has one hundred and fifty-seven patent family members in twenty-nine countries.
There are twenty-six drug master file entries for triamcinolone acetonide. Seventy-one suppliers are listed for this compound.
Summary for triamcinolone acetonide
| International Patents: | 157 |
| US Patents: | 7 |
| Tradenames: | 27 |
| Applicants: | 59 |
| NDAs: | 139 |
| Drug Master File Entries: | 26 |
| Finished Product Suppliers / Packagers: | 71 |
| Raw Ingredient (Bulk) Api Vendors: | 80 |
| Clinical Trials: | 262 |
| Patent Applications: | 7,040 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price trends for triamcinolone acetonide |
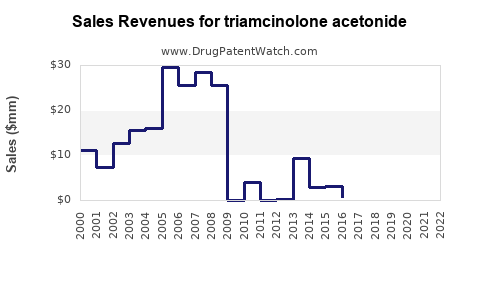
| Drug Sales Revenues: | Drug sales revenues for triamcinolone acetonide |
| What excipients (inactive ingredients) are in triamcinolone acetonide? | triamcinolone acetonide excipients list |
| DailyMed Link: | triamcinolone acetonide at DailyMed |
Recent Clinical Trials for triamcinolone acetonide
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Ain Shams University | Phase 3 |
| CHU de Reims | N/A |
| Tanta University | N/A |
Pharmacology for triamcinolone acetonide
| Drug Class | Corticosteroid |
| Mechanism of Action | Corticosteroid Hormone Receptor Agonists |
Medical Subject Heading (MeSH) Categories for triamcinolone acetonide
Anatomical Therapeutic Chemical (ATC) Classes for triamcinolone acetonide
US Patents and Regulatory Information for triamcinolone acetonide
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Taro | TRIAMCINOLONE ACETONIDE | triamcinolone acetonide | CREAM;TOPICAL | 086277-001 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Macleods Pharms Ltd | TRIAMCINOLONE ACETONIDE | triamcinolone acetonide | OINTMENT;TOPICAL | 209828-001 | Nov 23, 2018 | AT | RX | No | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| Sanofi Aventis Us | NASACORT HFA | triamcinolone acetonide | SPRAY, METERED;NASAL | 020784-001 | Apr 7, 2004 | DISCN | No | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Alpharma Us Pharms | TRIAMCINOLONE ACETONIDE | triamcinolone acetonide | LOTION;TOPICAL | 087191-001 | Sep 8, 1982 | DISCN | No | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Taro | TRIAMCINOLONE ACETONIDE | triamcinolone acetonide | PASTE;DENTAL | 070730-001 | Oct 1, 1986 | AT | RX | No | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| Astellas | ARISTOCORT A | triamcinolone acetonide | CREAM;TOPICAL | 083015-003 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for triamcinolone acetonide
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Abbvie | AZMACORT | triamcinolone acetonide | AEROSOL, METERED;INHALATION | 018117-001 | Apr 23, 1982 | ⤷ Sign Up | ⤷ Sign Up |
| Chattem Sanofi | NASACORT | triamcinolone acetonide | AEROSOL, METERED;NASAL | 019798-001 | Jul 11, 1991 | ⤷ Sign Up | ⤷ Sign Up |
| Chattem Sanofi | NASACORT ALLERGY 24 HOUR | triamcinolone acetonide | SPRAY, METERED;NASAL | 020468-002 | Oct 11, 2013 | ⤷ Sign Up | ⤷ Sign Up |
| Harrow Eye | TRIESENCE | triamcinolone acetonide | INJECTABLE;INTRAVITREAL | 022048-001 | Nov 29, 2007 | ⤷ Sign Up | ⤷ Sign Up |
| Delcor Asset Corp | KENALOG-H | triamcinolone acetonide | CREAM;TOPICAL | 086240-001 | Approved Prior to Jan 1, 1982 | ⤷ Sign Up | ⤷ Sign Up |
| Chattem Sanofi | NASACORT ALLERGY 24 HOUR | triamcinolone acetonide | SPRAY, METERED;NASAL | 020468-002 | Oct 11, 2013 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for triamcinolone acetonide
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Philippines | 12015500986 | METHODS AND DEVICES FOR THE TREATMENT OF OCULAR DISEASES IN HUMAN SUBJECTS | ⤷ Sign Up |
| Mexico | 353466 | CORTICOSTEROIDES PARA EL TRATAMIENTO DE DOLOR DE ARTICULACIONES. (CORTICOSTEROIDS FOR THE TREATMENT OF JOINT PAIN.) | ⤷ Sign Up |
| Eurasian Patent Organization | 034963 | СПОСОБ ЛЕЧЕНИЯ РАССТРОЙСТВА ЗАДНЕГО СЕГМЕНТА ГЛАЗА (METHOD OF TREATING A POSTERIOR SEGMENT OF THE EYE DISORDER) | ⤷ Sign Up |
| Japan | 6487848 | ⤷ Sign Up | |
| China | 101959519 | Low viscosity, highly flocculated triamcinolone acetonide suspensions for intravitreal injection | ⤷ Sign Up |
| European Patent Office | 2563429 | APPAREIL POUR L'ADMINISTRATION D'UN MÉDICAMENT À UN TISSU OCULAIRE À L'AIDE D'UNE MICRO-AIGUILLE (DEVICE FOR DELIVERY OF A DRUG TO OCULAR TISSUE USING MICRONEEDLE) | ⤷ Sign Up |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.