Janssen Pharms Company Profile
✉ Email this page to a colleague
What is the competitive landscape for JANSSEN PHARMS, and what generic alternatives to JANSSEN PHARMS drugs are available?
JANSSEN PHARMS has fifty-eight approved drugs.
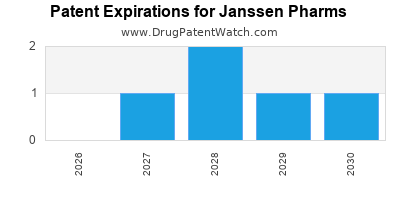
There are twenty-nine US patents protecting JANSSEN PHARMS drugs.
There are seven hundred and thirty-two patent family members on JANSSEN PHARMS drugs in sixty-five countries and one hundred and two supplementary protection certificates in eighteen countries.
Summary for Janssen Pharms
| International Patents: | 732 |
| US Patents: | 29 |
| Tradenames: | 57 |
| Ingredients: | 35 |
| NDAs: | 58 |
Drugs and US Patents for Janssen Pharms
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Janssen Pharms | INVOKAMET | canagliflozin; metformin hydrochloride | TABLET;ORAL | 204353-003 | Aug 8, 2014 | RX | Yes | No | 11,576,894 | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Janssen Pharms | INVEGA HAFYERA | paliperidone palmitate | SUSPENSION, EXTENDED RELEASE;INTRAMUSCULAR | 207946-005 | Aug 30, 2021 | RX | Yes | No | 11,304,951 | ⤷ Sign Up | ⤷ Sign Up | ||||
| Janssen Pharms | PONVORY | ponesimod | TABLET;ORAL | 213498-009 | Mar 18, 2021 | RX | Yes | No | 9,000,018 | ⤷ Sign Up | ⤷ Sign Up | ||||
| Janssen Pharms | PONVORY | ponesimod | TABLET;ORAL | 213498-003 | Mar 18, 2021 | RX | Yes | No | 9,062,014 | ⤷ Sign Up | Y | Y | ⤷ Sign Up | ||
| Janssen Pharms | PONVORY | ponesimod | TABLET;ORAL | 213498-006 | Mar 18, 2021 | RX | Yes | No | 8,273,779 | ⤷ Sign Up | ⤷ Sign Up | ||||
| Janssen Pharms | PONVORY | ponesimod | TABLET;ORAL | 213498-005 | Mar 18, 2021 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | |||||
| Janssen Pharms | PONVORY | ponesimod | TABLET;ORAL | 213498-007 | Mar 18, 2021 | RX | Yes | No | 9,062,014 | ⤷ Sign Up | Y | Y | ⤷ Sign Up | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Janssen Pharms
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Janssen Pharms | RISPERDAL | risperidone | TABLET, ORALLY DISINTEGRATING;ORAL | 021444-003 | Apr 2, 2003 | 4,804,663*PED | ⤷ Sign Up |
| Janssen Pharms | INVEGA SUSTENNA | paliperidone palmitate | SUSPENSION, EXTENDED RELEASE;INTRAMUSCULAR | 022264-004 | Jul 31, 2009 | 6,555,544*PED | ⤷ Sign Up |
| Janssen Pharms | TOPAMAX SPRINKLE | topiramate | CAPSULE;ORAL | 020844-003 | Oct 26, 1998 | 7,125,560*PED | ⤷ Sign Up |
| Janssen Pharms | RISPERDAL CONSTA | risperidone | INJECTABLE;INTRAMUSCULAR | 021346-002 | Oct 29, 2003 | 5,688,801*PED | ⤷ Sign Up |
| Janssen Pharms | LEVAQUIN | levofloxacin | INJECTABLE;INJECTION | 020635-004 | Dec 20, 1996 | 5,053,407*PED | ⤷ Sign Up |
| Janssen Pharms | RAZADYNE | galantamine hydrobromide | TABLET;ORAL | 021169-003 | Feb 28, 2001 | 6,099,863 | ⤷ Sign Up |
| Janssen Pharms | TOPAMAX | topiramate | TABLET;ORAL | 020505-002 | Dec 24, 1996 | 4,513,006*PED | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for JANSSEN PHARMS drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Tablets | 100 mg and 300 mg | ➤ Subscribe | 2017-03-29 |
| ➤ Subscribe | Oral Solution | 10 mg/mL | ➤ Subscribe | 2013-05-03 |
| ➤ Subscribe | Tablets | 50 mg | ➤ Subscribe | 2005-09-08 |
| ➤ Subscribe | Transdermal System | 0.15 mg/0.02 mg per 24 hours | ➤ Subscribe | 2007-03-22 |
| ➤ Subscribe | Oral Solution | 25 mg/mL | ➤ Subscribe | 2009-07-30 |
| ➤ Subscribe | Orally Disintegrating Tablets | 0.25 mg | ➤ Subscribe | 2005-04-11 |
| ➤ Subscribe | Extended-release Injectable Suspension | 39 mg/0.25 mL,78 mg/0.5 mL,117 mg/0.75 mL,156 mg/mL and234 mg/1.5 mL | ➤ Subscribe | 2017-11-21 |
| ➤ Subscribe | Extended-release Tablets | 18 mg*, 27 mg, 36 mg and 54 mg | ➤ Subscribe | 2005-07-19 |
| ➤ Subscribe | Tablets | 50 mg/500 mg, 50mg/1000 mg, 150mg/500 mg, and150 mg/1000 mg | ➤ Subscribe | 2017-03-29 |
| ➤ Subscribe | Extended-release Capsules | 16 mg and 24 mg | ➤ Subscribe | 2006-03-11 |
| ➤ Subscribe | Tablets | 25 mg, 100 mg and 200 mg | ➤ Subscribe | 2001-12-26 |
| ➤ Subscribe | Capsules | 15 mg and 25 mg | ➤ Subscribe | 2005-09-07 |
| ➤ Subscribe | Tablets | 10 mg, 15 mg, and 20 mg | ➤ Subscribe | 2015-07-01 |
| ➤ Subscribe | Tablets | 4 mg, 8 mg and 12 mg | ➤ Subscribe | 2005-02-28 |
| ➤ Subscribe | Orally Disintegrating Tablets | 3 mg and 4 mg | ➤ Subscribe | 2005-03-23 |
| ➤ Subscribe | Extended-release Tablets | 18 mg, 27 mg, 36 mg and 54 mg | ➤ Subscribe | 2005-07-19 |
| ➤ Subscribe | Tablets | 6.25 mg and 12.5 mg | ➤ Subscribe | 2005-12-08 |
| ➤ Subscribe | Extended-release Capsules | 8 mg | ➤ Subscribe | 2006-03-02 |
International Patents for Janssen Pharms Drugs
Supplementary Protection Certificates for Janssen Pharms Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1453521 | 93156 | Luxembourg | ⤷ Sign Up | PRODUCT NAME: LEVONORGESTREL ET ETHINYLESTRADIOL; FIRST REGISTRATION DATE: 20150211 |
| 3256125 | 22C1021 | France | ⤷ Sign Up | PRODUCT NAME: PONESIMOD ET SES SELS PHARMACEUTIQUEMENT ACCEPTABLES; REGISTRATION NO/DATE: EU/1/21/1550 20210521 |
| 0368388 | SPC/GB07/065 | United Kingdom | ⤷ Sign Up | PRODUCT NAME: PALIPERIDONE, OPTIONALLY IN THE FORM OF A PHARMACEUTICALLY ACCEPTABLE SALT; REGISTERED: UK EU/1/07/395/001 20070625; UK EU/1/07/395/002 20070625; UK EU/1/07/395/003 20070625; UK EU/1/07/395/004 20070625; UK EU/1/07/395/005 20070625; UK EU/1/07/395/006 20070625; UK EU/1/07/395/007 20070625; UK EU/1/07/395/008 20070625; UK EU/1/07/395/009 20070625; UK EU/1/07/395/010 20070625; UK EU/1/07/395/011 20070625; UK EU/1/07/395/012 20070625; UK EU/1/07/395/013 20070625; UK EU/1/07/395/014 20070625; UK EU/1/07/395/015 20070625; UK EU/1/07/395/016 20070625; UK EU/1/07/395/017 20070625; UK EU/1/07/395/018 20070625; UK EU/1/07/395/019 20070625; UK EU/1/07/395/020 20070625; UK EU/1/07/395/021 |
| 1429780 | 122012000070 | Germany | ⤷ Sign Up | PRODUCT NAME: KOMBINATION AUS CIPROFLOXACIN UND DEXAMETHASON, INSBESONDERE CIPROFLOXACINHYDROCHLORID UND DEXAMETHASON; NAT. REGISTRATION NO/DATE: 85150.00. 00 20120830; FIRST REGISTRATION: DAENEMARK 48976 20120808 |
| 2805723 | LUC00064 | Luxembourg | ⤷ Sign Up | PRODUCT NAME: CLADRIBINE; AUTHORISATION NUMBER AND DATE: EU/1/17/1212 20170824 |
| 0771217 | CA 2006 00038 | Denmark | ⤷ Sign Up | PRODUCT NAME: ETHINYLESTRADIOL (SOM BETA-CYCLODEXTRIN-CLATHRAT) OG DROSPIRENON; NAT. REG. NO/DATE: 38687 20060627; FIRST REG. NO/DATE: EU RVG 31781 20050804 |
| 0413455 | 98C0040 | Belgium | ⤷ Sign Up | PRODUCT NAME: ALATROFLOXACIN; NAT. REGISTRATION NO/DATE: EU/1/98/060/001 19980327; FIRST REGISTRATION: CH 54484 19980327 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.