Galderma Labs Lp Company Profile
✉ Email this page to a colleague
What is the competitive landscape for GALDERMA LABS LP, and when can generic versions of GALDERMA LABS LP drugs launch?
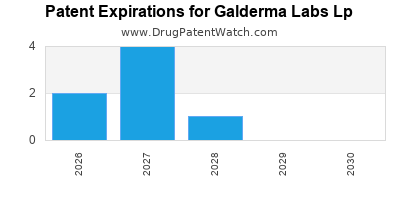
GALDERMA LABS LP has twenty-six approved drugs.
There are fifty-eight US patents protecting GALDERMA LABS LP drugs.
There are four hundred and five patent family members on GALDERMA LABS LP drugs in thirty-seven countries and one hundred and seventy-eight supplementary protection certificates in eighteen countries.
Summary for Galderma Labs Lp
| International Patents: | 405 |
| US Patents: | 58 |
| Tradenames: | 18 |
| Ingredients: | 16 |
| NDAs: | 26 |
Drugs and US Patents for Galderma Labs Lp
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Galderma Labs Lp | SOOLANTRA | ivermectin | CREAM;TOPICAL | 206255-001 | Dec 19, 2014 | AB | RX | Yes | Yes | 9,089,587 | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Galderma Labs Lp | AKLIEF | trifarotene | CREAM;TOPICAL | 211527-001 | Oct 4, 2019 | RX | Yes | Yes | 9,498,465 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Galderma Labs Lp | ORACEA | doxycycline | CAPSULE;ORAL | 050805-001 | May 26, 2006 | RX | Yes | Yes | 7,749,532 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Galderma Labs Lp | DESOWEN | desonide | LOTION;TOPICAL | 072354-001 | Jan 24, 1992 | AB | RX | No | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Galderma Labs Lp | METROCREAM | metronidazole | CREAM;TOPICAL | 020531-001 | Sep 20, 1995 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Galderma Labs Lp | TRI-LUMA | fluocinolone acetonide; hydroquinone; tretinoin | CREAM;TOPICAL | 021112-001 | Jan 18, 2002 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Galderma Labs Lp | EPSOLAY | benzoyl peroxide | CREAM;TOPICAL | 214510-001 | Apr 22, 2022 | RX | Yes | Yes | 10,933,046 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Galderma Labs Lp
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Galderma Labs Lp | ORACEA | doxycycline | CAPSULE;ORAL | 050805-001 | May 26, 2006 | 7,232,572 | ⤷ Try a Trial |
| Galderma Labs Lp | DIFFERIN | adapalene | SOLUTION;TOPICAL | 020338-001 | May 31, 1996 | 4,717,720 | ⤷ Try a Trial |
| Galderma Labs Lp | EPIDUO | adapalene; benzoyl peroxide | GEL;TOPICAL | 022320-001 | Dec 8, 2008 | 4,717,720 | ⤷ Try a Trial |
| Galderma Labs Lp | METROGEL | metronidazole | GEL;TOPICAL | 021789-001 | Jun 30, 2005 | 6,881,726 | ⤷ Try a Trial |
| Galderma Labs Lp | METVIXIA | methyl aminolevulinate hydrochloride | CREAM;TOPICAL | 021415-001 | Jul 27, 2004 | 6,034,267 | ⤷ Try a Trial |
| Galderma Labs Lp | EPIDUO | adapalene; benzoyl peroxide | GEL;TOPICAL | 022320-001 | Dec 8, 2008 | 8,105,618 | ⤷ Try a Trial |
| Galderma Labs Lp | ORACEA | doxycycline | CAPSULE;ORAL | 050805-001 | May 26, 2006 | 5,919,775 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for GALDERMA LABS LP drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Gel | 0.1%/2.5% | ➤ Subscribe | 2011-12-30 |
| ➤ Subscribe | Topical Gel | 0.33% | ➤ Subscribe | 2014-12-15 |
| ➤ Subscribe | Spray | 0.05% | ➤ Subscribe | 2008-09-29 |
| ➤ Subscribe | Delayed-release Capsules | 40 mg | ➤ Subscribe | 2008-12-11 |
| ➤ Subscribe | Cream | 1% | ➤ Subscribe | 2016-12-30 |
| ➤ Subscribe | Topical Gel | 1% | ➤ Subscribe | 2008-10-21 |
| ➤ Subscribe | Lotion | 0.05% | ➤ Subscribe | 2006-03-27 |
| ➤ Subscribe | Topical Shampoo | 0.05% | ➤ Subscribe | 2008-01-09 |
| ➤ Subscribe | Topical Gel | 0.30% | ➤ Subscribe | 2009-09-15 |
International Patents for Galderma Labs Lp Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Canada | 2792646 | ⤷ Try a Trial |
| Slovenia | 1831149 | ⤷ Try a Trial |
| South Korea | 20060004667 | ⤷ Try a Trial |
| South Africa | 200403759 | ⤷ Try a Trial |
| Mexico | PA04005918 | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 2020170029 | ⤷ Try a Trial |
| Slovenia | 1631293 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Galderma Labs Lp Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2187879 | SPC/GB17/031 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: 1-CHLORO-4-(SS-D-GLUCOPYRANOS-1-YL)-2-(4-((S)-TETRAHYDROFURAN-3-YLOXY)-BENZYL)-BENZENE (I.E. EMPAGLIFLOZIN) IN COMBINATION WITH 1-((4 METHYL-QUINAZOLIN-2-YL)METHYL)-3-METHYL-7-(2-BUTYN-1-YL)-8-(3-(R)-AMINO-PIPERIDIN-1-YL)-XANTHINE (I.E. LINAGLIPTIN) OR A P; REGISTERED: UK PLGB 14598/0191 (GB) 20161115; UK EU/1/16/1146/014(NI) 20161115; UK EU/1/16/1146/015(NI) 20161115; UK EU/1/16/1146/016(NI) 20161115; UK EU/1/16/1146/017(NI) 20161115; UK EU/1/16/1146/018(NI) 20161115; UK EU/1/16/1146/007(NI) 20161115; UK EU/1/16/1146/001(NI) 20161115; UK EU/1/16/1146/013(NI) 20161115; UK EU/1/16/1146/002(NI) 20161115; UK EU/1/16/1146/003(NI) 20161115; UK EU/1/16/1146/004(NI) 20161115; UK... |
| 1948158 | 93075 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: SACUBITRIL ET VALSARTAN, SOUS FORME DE COMPLEXE SODIQUE SACUBITRIL VALSARTAN, C'EST-A-DIRE DE (3-((1S,3R)-1-BIPHENYL-4-YLMETHYL-3-ETHOXYCARBONYL-1-BUTYLCARBAMOYL)PROPIONATE-(S)-3'-METHYL-2-(PENTANYOL(2''-(TETRAZOL-5-YLATE)BIPHENYL-4'-YLMETHYL)AMINO)BUTYRATE) DE TRISODIUM HEMIPENTAHYDRATE; AUTHORISATION NUMBER AND DATE: EU/1/15/1058 20151123 |
| 1458369 | 08C0024 | France | ⤷ Try a Trial | PRODUCT NAME: ADAPALENE - PEROXYDE DE BENZOLE; REGISTRATION NO/DATE IN FRANCE: NL 33724 DU 20080123; REGISTRATION NO/DATE AT EEC: 40440 DU 20071218 |
| 0252504 | C960032 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: ACIDUM IBANDRONICUM, DESGEWENST IN DE VORM VAN EEN FARMACOLO- GISCH AANVAARDBAAR ZOUT OF IN DE VORM VAN EEN ESTER MET METHANOL,ETHANOL,2-PROPANOL OF 2-METHYLPROPANOL, OF IN DE VORM VEN EEN HYDRAAT,I.H.B.MONONATRIUM IBANDRONAAT MONOHYDRAAT; REGISTRATION NO/DATE: EU/1/96/012/001 19960625 |
| 1631293 | CR 2014 00031 | Denmark | ⤷ Try a Trial | PRODUCT NAME: BRIMONIDIN OG ET FARMACEUTISK ACCEPTABELT SALT DERAF, HERUNDER BRIMONIDIN TARTRAT; REG. NO/DATE: EU/1/13/904/001-003 20140221 |
| 1948158 | 1690020-1 | Sweden | ⤷ Try a Trial | PRODUCT NAME: SACUBITRIL AND VALSARTAN, AS SACUBITRIL VALSARTAN SODIUM SALT COMPLEX, I.E. TRISODIUM 3-((1S,3R)-1-BIPHENYL-4-YLMETHYL-3- ETHOXYCARBONYL-1-BUTYLCARBAMOYL)PROPIONATE-(S)-3-METHYL-2- (PENTANOYL2-(TETRAZOL-5-YLATE)BIPHENYL-4- YLMETHYLAMINO)BUTYRATE HEMIPENTAHYDRATE; REG. NO/DATE: EU/1/15/1058 20151123 |
| 2404919 | 122016000035 | Germany | ⤷ Try a Trial | PRODUCT NAME: 3-(6-((1-(2,2-DIFLUOR-1,3-BENZODIOXOL-5-YL)CYCLOPROPANCARBONYL)AMINO)-3-METHYLPYRIDIN-2-YL)BENZOESAEURE, ODER EIN PHARMAZEUTISCH ANNEHMBARES SALZ DAVON, ODER EIN ESTER-PRODRUG DAVON.; REGISTRATION NO/DATE: EU/1/15/1059 20151119 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.