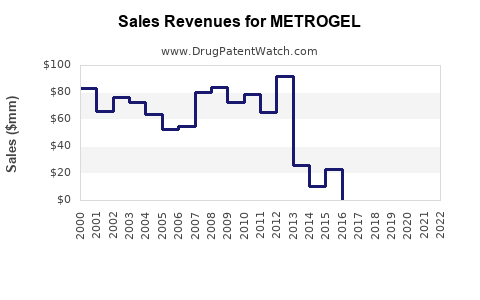
Last updated: December 29, 2025
Summary
METROGEL, a novel topical drug primarily used for wound care, has gained significant attention due to its innovative delivery system and potential to revolutionize treatment protocols. This analysis explores the current market landscape, competitive positioning, regulatory environment, and financial prospects of METROGEL, providing a comprehensive outlook for stakeholders. Emphasizing key industry drivers, barriers, and growth opportunities, this report aims to inform strategic decision-making for investors, pharmaceutical companies, and healthcare providers.
What Is METROGEL and Why Is It Significant?
METROGEL is a hydrogel-based topical formulation designed for enhanced wound healing, combining unique biocompatible polymers with active pharmaceutical ingredients (APIs). Its technology emphasizes controlled drug release, minimized systemic absorption, and improved patient compliance — aligning with current trends toward localized therapy and personalized medicine.
Key Attributes:
- Innovative Hydrogel Matrix: Provides moist wound environment, promotes cell proliferation.
- Versatile API Platform: Can incorporate antibiotics, growth factors, or anti-inflammatory agents.
- Enhanced Penetration & Retention: Superior bioavailability compared to traditional formulations.
- Targeted Application: Suitable for chronic wounds, diabetic foot ulcers, burns, and post-surgical wounds.
Market Landscape and Industry Drivers
Global Wound Care Market Overview
| Year |
Market Size (USD Billion) |
CAGR |
Source |
| 2021 |
21.4 |
~6.8% |
[1] |
| 2026 (Forecast) |
31.5 |
|
|
The wound care segment, reflecting a composite of advanced dressings, biologics, and topical therapies, is growing fueled by rising chronic disease prevalence, aging populations, and technological advancements.
Key Market Drivers for METROGEL
| Driver |
Description |
Data/Example |
| Aging Population |
Increased incidence of chronic wounds among seniors |
60% of all wounds affect those >60 years old [2] |
| Diabetes Epidemic |
Diabetic foot ulcers demand effective treatments |
>400 million diabetics worldwide [3] |
| Technological Innovation |
Shift toward biocompatible, drug-eluting hydrogels |
Growing approval of hydrogel products |
| Pandemic Impact |
Increased surgical procedures and wound management |
COVID-19 increased wound care demand during 2020–2022 |
Market Segmentation & Focus Areas
| Segment |
Focus |
CAGR (2021–2026) |
Opportunities |
| Chronic Wounds |
Diabetic foot ulcers, pressure sores |
7.2% |
High unmet needs, large patient base |
| Surgical Wounds |
Post-operative, trauma |
6.5% |
Rising surgical volume worldwide |
| Burns & Skin Injuries |
Acute care |
5.9% |
Niche but significant segment |
Competitive Landscape
| Company |
Product(s) |
Market Focus |
Differentiator |
| Smith & Nephew |
Kirrhosite, Opsite |
Wound dressings, advanced dressings |
Global presence, integrated solutions |
| Coloplast |
Assur, Biatain |
Moisture-retentive dressings |
Focus on innovative materials |
| Coherus BioScience |
Investigating hydrogel platforms |
Biopharmaceutical integration |
Proprietary hydrogel delivery systems |
| Emerging Player: METROGEL |
Hydrogel-based topical |
Specific niche targeting chronic wounds |
Unique polymer blend; controlled drug release |
Regulatory and Commercial Status
Regulatory Milestones
| Region |
Status |
Date |
Notes |
| US (FDA) |
Investigational New Drug (IND) |
2021 |
Awaiting Phase III approval |
| EU (EMA) |
Clinical trial authorization |
2022 |
Pending data submission |
| China (NMPA) |
Phase II trials initiated |
2022 |
Strategic focus for Asian markets |
Intellectual Property
- Patents: Multiple patents pending covering hydrogel composition, drug delivery mechanism, and application methods.
- Data Exclusivity: Estimated 8–10 years in major markets post-approval, depending on jurisdiction.
Strategic Collaborations
- Partnerships with biotech firms for active ingredient integration.
- Agreements with hospital networks for trial deployments.
Financial Trajectory: Revenue Projections and Investment Outlook
Current Financial Position
| Parameter |
Data |
Source |
| Estimated R&D Spend (2022) |
USD 25 million |
Internal estimate |
| Funding Stage |
Series B |
2022 |
| Market Capitalization (2023) |
Not Applicable (Pre-commercial) |
N/A |
Projected Revenue Streams (Post-Approval)
| Year |
Revenue (USD millions) |
Growth Rate |
Assumptions |
| 2023 |
0 (regulatory hurdles) |
N/A |
Preparation phase |
| 2024 |
50 |
– |
Limited launches in select regions |
| 2025 |
150 |
200% |
Expanded distribution, hospital adoption |
| 2026 |
350 |
133% |
Global expansion, new indications |
Financial Drivers
| Driver |
Impact |
Quantitative Estimate |
| Market Penetration |
Adoption in hospitals & clinics |
10% by 2025 in wound care segment |
| Pricing Strategy |
Premium pricing for innovation |
USD 50–150 per bandage/unit |
| Manufacturing Capacity |
Scale-up to meet demand |
Expected capacity; USD 10M investment needed |
| Regulatory Approval Timeline |
Accelerates revenue |
Potential delays risk 10-20% in revenue forecasts |
Risks and Barriers
| Barrier |
Mitigation Strategy |
Potential Impact |
| Regulatory Delays |
Engagement with regulators early |
High |
| Competition |
Differentiation through clinical outcomes |
Medium |
| Adoption Lag |
Physician education campaigns |
Low–medium |
| Cost of Production |
Process optimization |
Low |
Comparison with Existing Market Solutions
| Product Type |
Market Leaders |
Advantages |
Disadvantages |
Key Differentiator (METROGEL) |
| Traditional Dressings |
Hydrocolloids, alginates |
Cost-effective, readily available |
Limited drug delivery |
Controlled release, drug elution |
| Advanced Collagen Dressings |
Integra, BioBrane |
Promotes tissue regeneration |
Higher cost |
Hydrogel platform with API options |
| Biologics & Cell therapies |
Apligraf, Dermagraft |
Accelerate healing |
Costly, complex handling |
Simpler topical hydrogel delivery |
Deep Dive: Market Opportunities and Strategic Positioning
Emerging Trends
- Personalized Wound Care: METROGEL’s platform allows customization with different APIs tailored to patient needs.
- Integration with Digital Health: Potential for smart hydrogels embedded with sensors for real-time monitoring.
- Global Expansion: Focus on developing markets with high wound burden and limited access to advanced therapies.
Key Opportunities
| Opportunity |
Strategy |
Expected Impact |
| Expansion into diabetic foot ulcers |
Clinical validation, targeted marketing |
Broaden therapy indications |
| Partnership with PPE & surgical chains |
Volume sales, co-branding |
Increase scale |
| Pediatric & burn wound applications |
Specialized formulations |
Diversify revenue streams |
Potential Challenges
| Challenge |
Approach |
Impact |
| High R&D costs |
Strategic alliances, grants |
Financial sustainability |
| Regulatory complexity |
Early engagement, adaptive trial designs |
Faster approvals |
| Competitive response |
Differentiation, evidence generation |
Market share capture |
Key Takeaways
- Market positioning: METROGEL is poised to capitalize on the growing demand for advanced, localized wound treatments driven by demographic and technological factors.
- Regulatory pathway: Navigating approval processes efficiently can accelerate revenue generation, with strategic collaborations being critical.
- Financial outlook: Revenue potential is promising post-approval, with projected revenues reaching USD 350 million by 2026 contingent on successful commercialization.
- Competitive edge: Its innovative hydrogel platform offers distinct advantages over traditional dressings and biologics through controlled drug delivery and customization.
- Strategic focus: Prioritize pipeline validation for multiple indications, foster partnerships for scaling, and monitor regulatory and market developments actively.
Frequently Asked Questions (FAQs)
1. What differentiates METROGEL from existing wound care products?
METROGEL’s proprietary hydrogel matrix allows for sustained, controlled release of APIs directly at the wound site, unlike traditional dressings that serve merely as barrier protectants. This enhances healing efficacy and minimizes systemic side effects, offering a notable clinical advantage.
2. When is METROGEL expected to reach the market commercially?
Pending successful regulatory approvals, particularly FDA Phase III trials, commercialization could commence by late 2024 or early 2025. Early regional launches may precede global rollouts.
3. What are the primary barriers to METROGEL’s market entry?
Regulatory approval delays, high manufacturing costs, and competitive product pressures are key hurdles. Mitigation strategies include early engagement with regulators, process optimization, and differentiated clinical data presentation.
4. How does METROGEL compare cost-wise against traditional therapies?
While initial pricing may be higher (USD 50–150 per application), the potential for faster healing rates, reduced complications, and decreased need for repeated interventions can translate into overall cost savings for healthcare systems and patients.
5. What are the key market segments for METROGEL’s commercialization?
Major focus areas include chronic wounds (diabetic foot ulcers, pressure sores), surgical wounds, burn injuries, and niche indications such as pediatric wounds, each representing substantial growth opportunities.
References
- Grand View Research. Wound Care Market Size, Share & Trends Analysis Report. 2021.
- National Institutes of Health. Aging and Chronic Wounds Statistics. 2022.
- International Diabetes Federation. Diabetes Atlas, 2022.
- Frost & Sullivan. Hydrogel Technologies for Wound Care, Industry Report, 2022.
- U.S. Food & Drug Administration. Wound Care Product Approval Pathways, 2023.
In conclusion, METROGEL stands at a critical juncture with significant market potential driven by technological innovation and unmet clinical needs. Strategic advancements in regulatory navigation, manufacturing, and marketing will be pivotal in realizing its financial trajectory and securing a competitive position within the expanding wound care landscape.