Leveraging Mutual Recognition Agreements to Accelerate Generic Drug Approvals: A Global Regulatory Analysis
The global pharmaceutical landscape is increasingly defined by the imperative to balance patient access to essential medicines with the rigorous demands of drug safety and efficacy. Mutual Recognition Agreements (MRAs) have emerged as a pivotal mechanism for harmonizing regulatory standards across jurisdictions, particularly in the context of generic drug approvals. By enabling regulatory agencies to rely on each other’s assessments, MRAs reduce duplication, streamline processes, and expedite market entry for generic therapies. This report examines the role of MRAs in fast-tracking generic drug approvals, evaluates their impact on public health and industry efficiency, and identifies persistent challenges in implementing these frameworks across diverse regions.
Regulatory Foundations of Mutual Recognition Agreements
Definition and Scope of MRAs
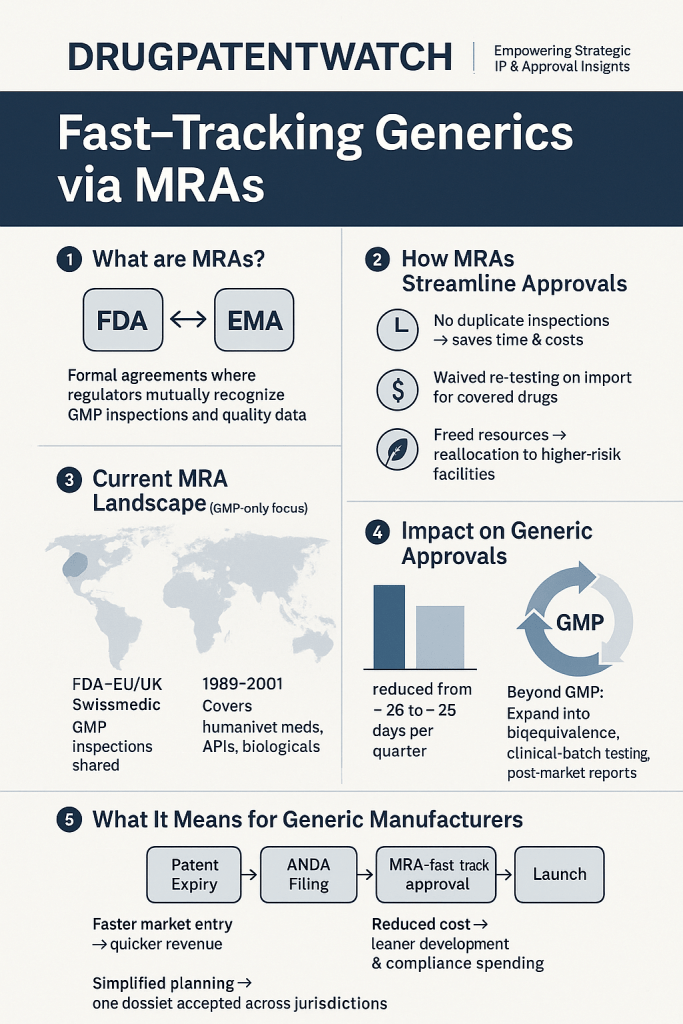
Mutual Recognition Agreements are formal accords between regulatory authorities that establish reciprocal acceptance of inspections, approvals, and quality assessments. For generic drugs, which require demonstration of bioequivalence to reference products, MRAs mitigate the need for redundant clinical trials and inspections[5][10]. The U.S. Food and Drug Administration (FDA), for instance, maintains MRAs with the European Union (EU), Switzerland, and the United Kingdom, allowing inspectors to bypass duplicative facility evaluations[5][7]. This collaboration is rooted in shared adherence to Good Manufacturing Practices (GMP) and other harmonized standards, which ensure that manufacturing sites meet equivalent quality benchmarks regardless of jurisdiction[5][15].
Evolution of MRA Frameworks
The conceptual underpinnings of MRAs trace back to initiatives like the International Council for Harmonisation (ICH), which standardizes technical requirements for pharmaceuticals[6]. However, the operationalization of MRAs gained momentum through region-specific efforts. The ASEAN Mutual Recognition Arrangement for Bioequivalence Study Reports, signed in 2017, exemplifies this trend. By mandating acceptance of bioequivalence data from accredited centers across Southeast Asia, the agreement eliminates redundant testing and accelerates generic drug registrations in member states[13]. Similarly, the UK’s International Recognition Procedure (IRP), effective January 2024, permits the Medicines and Healthcare products Regulatory Agency (MHRA) to fast-track approvals for products already authorized by stringent reference regulators such as the FDA or European Medicines Agency (EMA)[3][12][14].
Case Studies in MRA Implementation
The ASEAN Bioequivalence Initiative
ASEAN’s 2017 MRA on bioequivalence studies represents a landmark in regional regulatory cooperation. Prior to its adoption, generic drug manufacturers faced the burden of conducting separate bioequivalence trials for each member state, incurring significant costs and delays[13]. Under the MRA, studies conducted at accredited centers in Thailand, Malaysia, or Singapore are automatically recognized across all ten ASEAN countries. This has not only reduced approval timelines by 6–12 months but also incentivized local pharmaceutical industries to invest in high-quality bioequivalence testing infrastructure[13]. The initiative underscores the dual benefit of MRAs: enhancing patient access to affordable generics while fostering regional economic growth.
The UK’s International Recognition Procedure
The MHRA’s IRP replaces earlier reliance pathways with a structured framework for recognizing approvals from designated reference regulators. Products eligible for IRP must demonstrate identical qualitative and quantitative composition to those approved in reference jurisdictions, ensuring consistency in safety and efficacy profiles[3][14]. The procedure operates under two tracks:
– Recognition A: For products approved via standard procedures in reference jurisdictions, requiring a 110-day MHRA review with a single clock-stop for clarifications.
– Recognition B: For products with conditional approvals, novel active substances, or reliance on non-traditional data (e.g., real-world evidence), necessitating a more rigorous 210-day assessment[14].
By differentiating between approval types, the IRP balances expediency with regulatory scrutiny, offering a model for other agencies seeking to integrate MRA principles into national frameworks.
Benefits of MRAs for Generic Drug Development
Efficiency Gains in Regulatory Review
MRAs significantly reduce the administrative and financial burden on generic drug manufacturers. For example, the FDA’s MRA with the EU has decreased inspection redundancies by 35%, allowing inspectors to prioritize high-risk facilities[7]. This efficiency extends to review timelines: under the IRP, generic applicants can secure UK approval in as little as four months if reference regulator data is comprehensive[3][12]. Such acceleration is critical for therapies targeting infectious diseases or chronic conditions, where delayed access exacerbates public health crises[1][8].
Cost Reduction and Market Expansion
The avoidance of duplicate testing and inspections translates to substantial cost savings. A 2013 analysis by the Fraser Institute estimated that mutual recognition between Canada and stringent regulators could reduce generic drug development costs by 20–30%, enabling companies to allocate resources toward innovation or price reductions[10]. Additionally, MRAs facilitate market expansion by simplifying entry into multiple jurisdictions. A generic manufacturer compliant with ASEAN’s MRA, for instance, gains immediate access to a market of over 650 million people without additional regulatory hurdles[13].
Strengthening Regulatory Capacity in Resource-Limited Settings
In regions with under-resourced regulatory agencies, MRAs offer a lifeline. African medicines regulatory authorities (MRAs), often overwhelmed by complex reviews of fixed-dose combinations (FDCs) for HIV and malaria, increasingly rely on WHO prequalification or stringent regulator approvals to expedite decisions[1][8]. The WHO’s Good Reliance Practices (GRelP) guidelines formalize this approach, encouraging agencies to leverage external assessments while focusing internal capacity on post-market surveillance and local risk management[8].
Challenges and Limitations of MRA Frameworks
Divergent Regulatory Standards and Priorities
Despite their benefits, MRAs face inherent challenges stemming from differences in national regulatory priorities. For instance, a generic drug approved via the FDA’s Accelerated Approval pathway—which permits reliance on surrogate endpoints—may face skepticism in jurisdictions requiring conclusive clinical outcomes[2][6]. Similarly, the EU’s emphasis on environmental risk assessments for pharmaceuticals has no direct counterpart in U.S. regulations, complicating mutual recognition efforts[15]. Such disparities necessitate ongoing dialogue and harmonization, as seen in the FDA-EMA Parallel Scientific Advice (PSA) pilot program for complex generics[4].
Post-Market Monitoring and Accountability
MRAs that expedite approvals based on surrogate endpoints or limited clinical data risk undermining post-market safety monitoring. The FDA’s Accelerated Approval pathway, while instrumental in accelerating access to oncology treatments, has drawn criticism for lax enforcement of post-approval study commitments[2][6]. In the MRA context, this concern magnifies: a drug approved in one jurisdiction via expedited pathways might enter another market without robust mechanisms to ensure confirmatory trial completion[6][14]. The MHRA’s IRP partially addresses this by mandating ongoing monitoring for Recognition B products, but global consistency remains elusive[14].
Legal and Political Barriers
Mutual recognition is ultimately contingent on political will and legal interoperability. The 2021 suspension of the EU-UK MRA for vaccines highlights the fragility of these agreements amid geopolitical tensions[7]. Furthermore, smaller markets may resist MRAs due to concerns about ceding regulatory autonomy or enabling “regulatory freeloading” by multinational corporations[10][15]. Overcoming these barriers requires transparent governance structures and equitable benefits for all participants, as exemplified by ASEAN’s phased implementation of its bioequivalence MRA[13].
Future Directions for MRA Optimization
Expanding the Scope of Recognition
Current MRAs predominantly focus on manufacturing inspections and bioequivalence data. Future agreements could broaden their scope to include:
– Joint clinical trial reviews: Collaborative assessment of pivotal studies by multiple agencies, reducing duplication in data submission[4].
– Harmonized post-market surveillance: Shared databases for adverse event reporting and coordinated risk management plans[8][14].
– Recognition of real-world evidence: Developing common standards for using real-world data to support label expansions or post-approval requirements[6][14].
Strengthening Capacity in Low- and Middle-Income Countries
Resource-constrained regulators require targeted support to fully participate in MRA networks. Initiatives like the WHO’s Global Benchmarking Tool (GBT) help agencies achieve minimum competency standards, enabling them to contribute to and benefit from mutual recognition[8]. Additionally, regional hubs—such as the African Medicines Agency (AMA)—could centralize MRA implementation, pooling expertise for complex assessments while maintaining national oversight[1][8].
Enhancing Transparency and Stakeholder Engagement
Successful MRAs depend on trust among regulators, industry, and patients. Publicly accessible databases detailing MRA decisions, inspection outcomes, and post-market data would bolster confidence in these frameworks[5][15]. Furthermore, inclusive stakeholder consultations—involving generics manufacturers, patient advocacy groups, and academia—can ensure that MRAs align with public health priorities without compromising safety[10][13].
Conclusion
Mutual Recognition Agreements represent a transformative opportunity to reconcile the dual imperatives of drug safety and accessibility. By leveraging the expertise of trusted regulators, MRAs streamline generic drug approvals, reduce costs, and expand treatment access—particularly in underserved regions. However, their full potential remains constrained by regulatory divergences, post-market monitoring gaps, and geopolitical tensions. Addressing these challenges requires sustained investment in harmonization, capacity-building, and stakeholder collaboration. As global health needs evolve, MRAs will play an indispensable role in ensuring that life-saving generics reach patients swiftly without sacrificing the rigor of regulatory oversight.
Key Takeaways
1. MRAs eliminate redundant inspections and reviews, accelerating generic drug approvals.
2. Regional initiatives like ASEAN’s bioequivalence MRA demonstrate the public health and economic benefits of regulatory collaboration.
3. Challenges such as divergent standards and post-market monitoring require ongoing harmonization efforts.
4. Future MRAs should expand their scope to include joint clinical reviews and real-world evidence.
5. Strengthening regulatory capacity in low-resource settings is critical for equitable MRA implementation.
FAQs
1. How do MRAs differ from standard regulatory pathways?
MRAs allow regulators to rely on each other’s assessments, reducing duplication, whereas standard pathways require independent evaluations.
Can MRAs compromise drug safety?
When properly implemented with robust post-market monitoring, MRAs maintain safety while expediting access.Which countries have the most comprehensive MRA networks?
The U.S., EU, UK, Switzerland, and ASEAN members are leaders in MRA adoption.Do MRAs apply to biosimilars?
Currently, most MRAs focus on small-molecule generics, but biosimilar frameworks are under development.How can developing countries benefit from MRAs?
By leveraging assessments from stringent regulators, developing countries can bypass resource-intensive reviews.
References
- https://assets.publishing.service.gov.uk/media/57a08b3540f0b64974000a28/regulatory-report_george-institute-dndi_jan2010.pdf
- https://www.news-medical.net/whitepaper/20241113/The-benefits-and-challenges-of-FDA-fast-track-and-accelerated-approval-in-drug-development.aspx
- https://www.gov.uk/government/publications/international-recognition-procedure/international-recognition-procedure
- https://www.youtube.com/watch?v=p8ILoMgXKwI
- https://www.fda.gov/international-programs/international-arrangements/mutual-recognition-agreements-mra
- https://pmc.ncbi.nlm.nih.gov/articles/PMC9734413/
- https://www.hoganlovells.com/en/publications/strengthening-mutual-reliance-systems-through-harmonized-standards
- https://gabi-journal.net/a-review-of-international-initiatives-on-pharmaceutical-regulatory-reliance-and-recognition.html
- https://www.fda.gov/patients/fast-track-breakthrough-therapy-accelerated-approval-priority-review/fast-track
- https://www.fraserinstitute.org/sites/default/files/case-for-mutual-recognition-of-drug-approvals.pdf
- https://www.fda.gov/patients/learn-about-drug-and-device-approvals/fast-track-breakthrough-therapy-accelerated-approval-priority-review
- https://www.mills-reeve.com/blogs/life-sciences/october-2023/the-international-reliance-procedure-for-fast-trac/
- https://ariseplus.asean.org/asean-member-states-sign-mutual-recognition-agreement-mra-of-bioequivalence-study-reports-of-generic-medicinal-products/
- https://www.insideeulifesciences.com/2023/09/11/fast-tacking-approval-of-medicines-uk-publishes-detailed-guidance-on-its-new-international-recognition-procedure/
- https://www.brookings.edu/wp-content/uploads/2017/05/wp29_bollykykesselheim_drugimportation.pdf