Introduction
The pharmaceutical industry is a high-stakes arena where innovation meets stringent regulation. Developing a new drug can take over a decade and cost billions, with no guarantee of success. Patents are the lifeblood of this industry, protecting investments and granting companies exclusive rights to market their drugs. However, when patents expire, generic competition can erode market share, pushing companies to find creative ways to extend product lifecycles or introduce new therapies. Enter the 505(b)(2) pathway—a regulatory strategy that allows pharmaceutical companies to innovate efficiently by building on existing drugs, potentially bypassing the full scope of traditional development costs and timelines.
The 505(b)(2) pathway, established under the Hatch-Waxman Amendments of 1984, enables companies to seek approval for new drug applications (NDAs) by relying on data from previously approved drugs, even if they didn’t conduct those studies themselves. This approach is ideal for creating new formulations, indications, or combinations of existing drugs, offering a faster and less costly route to market. Importantly, it allows companies to innovate beyond existing patents, securing new intellectual property (IP) and market exclusivity to maintain a competitive edge.
This article explores how the 505(b)(2) pathway can be leveraged to innovate beyond existing drug patents, offering insights for business professionals in the pharmaceutical industry. We’ll delve into the mechanics of the pathway, its strategic advantages, real-world examples, and potential challenges, providing a comprehensive guide to turning patent data into a competitive advantage.
Understanding the 505(b)(2) Pathway
What is the 505(b)(2) Pathway?
The 505(b)(2) pathway is a type of New Drug Application (NDA) under the Federal Food, Drug, and Cosmetic Act (FD&C Act). Unlike a traditional 505(b)(1) NDA, which requires all safety and efficacy data to be generated by the applicant, a 505(b)(2) NDA allows reliance on existing data—such as published literature or the FDA’s prior findings for a reference listed drug (RLD)—without needing a right of reference. This pathway, codified under section 505(b)(2), is designed to avoid redundant studies, streamlining the approval process for drugs that differ from previously approved products.
For example, a company might use 505(b)(2) to gain approval for a new dosage form, a new indication, or a combination product based on an existing drug. The applicant must still provide sufficient data to demonstrate safety and efficacy, often through bridging studies that link the new product to the RLD.
“The 505(b)(2) pathway has become increasingly popular, with 60% of all approved NDAs in recent years being 505(b)(2) applications, and average review times dropping to just 10 months.” [1]
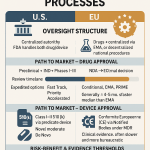
How It Differs from 505(b)(1) and ANDA
To appreciate the 505(b)(2) pathway, it’s helpful to compare it with other FDA approval routes:
- 505(b)(1) NDA: Used for novel drugs, requiring comprehensive preclinical and clinical studies conducted by or for the applicant. This pathway is time-intensive and costly, often taking 10–15 years and costing up to $1 billion [2].
- 505(b)(2) NDA: A hybrid approach for drugs that differ from an RLD but rely on existing data. It requires some new studies (e.g., bridging studies) but leverages prior data, reducing time and cost.
- ANDA (505(j)): For generic drugs, requiring bioequivalence to an RLD. Unlike 505(b)(2), ANDAs must demonstrate “sameness” in active ingredient, dosage form, strength, and route of administration, leaving little room for innovation.
The 505(b)(2) pathway strikes a balance, enabling innovation without the full burden of a 505(b)(1) NDA while offering more flexibility than an ANDA.
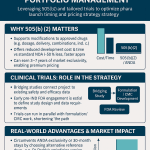
Benefits of the 505(b)(2) Pathway
The 505(b)(2) pathway offers several advantages for pharmaceutical companies:
- Cost and Time Savings: By leveraging existing data, companies can reduce development timelines by 5–10 years and costs significantly compared to 505(b)(1) [2].
- Market Exclusivity: Approved 505(b)(2) products may qualify for 3-year exclusivity for new clinical investigations, 5-year exclusivity for new chemical entities (NCEs), or 7-year exclusivity for orphan drugs [3].
- Innovation Opportunities: The pathway supports modifications like new formulations or indications, which can lead to new patents and market differentiation.
- Lower Risk: Since the active ingredient’s safety and efficacy are often established, the development risk is reduced compared to novel drugs.
These benefits make 505(b)(2) an attractive option for companies looking to innovate efficiently.
Innovating Beyond Existing Drug Patents
Types of Innovations Possible
The 505(b)(2) pathway enables a range of innovations that can extend a drug’s lifecycle or create new market opportunities. These include:
New Formulations
Developing novel delivery systems, such as extended-release tablets, transdermal patches, or nasal sprays, can improve patient convenience or efficacy. These formulations can be patented separately from the original drug.
New Indications
Seeking approval for a new therapeutic use can open new markets. For instance, a drug approved for one condition might be repurposed for another, potentially avoiding existing patents on the original use.
New Combinations
Combining an existing drug with another active ingredient can create a synergistic effect, addressing unmet needs and securing new IP.
New Dosage Forms
Changing the form (e.g., from tablet to liquid) can enhance patient compliance, especially for pediatric or geriatric populations.
New Strengths
Offering different dosages can cater to specific patient needs, potentially warranting new patents.
New Routes of Administration
Switching from oral to injectable or topical administration can improve bioavailability or patient experience, often leading to new IP.
Each of these innovations can result in new patents or exclusivity, allowing companies to differentiate their products and extend market presence.
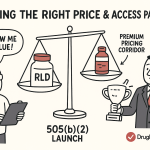
Patent Strategies
Navigating the patent landscape is critical when using 505(b)(2). Key strategies include:
Obtaining New Patents
Innovations like new formulations or indications can be patented, providing protection even after the RLD’s patents expire. For example, a novel delivery system might be eligible for a patent on its design or method of use.
Navigating Existing Patents
Companies must ensure their 505(b)(2) product does not infringe on existing patents. This may involve certifying to the FDA’s Orange Book patents for the RLD, potentially triggering litigation if a Paragraph IV certification (challenging patent validity or non-infringement) is filed [4].
Tools like DrugPatentWatch are invaluable here, offering detailed insights into patent expirations, exclusivity periods, and generic entry opportunities. By analyzing patent data, companies can identify gaps where new innovations won’t infringe and secure their own IP [5].
Market Exclusivity
Beyond patents, 505(b)(2) products can receive FDA-granted market exclusivity, which prevents competitors from gaining approval for similar products for a set period:
- 3-Year Exclusivity: For new clinical investigations (e.g., new indications or dosages) essential for approval [3].
- 5-Year Exclusivity: For NCEs, where the active moiety has not been previously approved [3].
- 7-Year Exclusivity: For orphan drugs treating rare diseases affecting fewer than 200,000 patients in the US [3].
- Pediatric Exclusivity: An additional 6 months added to existing exclusivity for conducting pediatric studies [3].
This exclusivity complements patents, providing a window to recoup development costs and establish market presence.
Case Studies
Emflaza (Deflazacort)
Overview: Emflaza, a corticosteroid for Duchenne muscular dystrophy (DMD), was approved in 2017 via 505(b)(2) by PTC Therapeutics. It relied on existing data from studies conducted outside the US and published literature, as deflazacort was used informally in the US through compassionate use programs.
Innovation: As a new molecular entity (NME) in the US, Emflaza addressed a rare disease, qualifying for 7-year orphan drug exclusivity. This allowed PTC to bring a critical treatment to market without replicating extensive studies.
Impact: The approval provided significant market protection, with DrugPatentWatch noting no US patents but two FDA exclusivities, extending protection until at least 2024 [6]. This case highlights how 505(b)(2) can formalize existing treatments for new markets.
Narcan (Naloxone Nasal Spray)
Overview: Approved in 2015, Narcan is a nasal spray formulation of naloxone, used to reverse opioid overdoses. It leveraged safety and efficacy data from injectable naloxone, focusing on the novel delivery system.
Innovation: The nasal spray improved ease of use in emergencies, earning patents for its delivery mechanism. This innovation addressed a public health crisis, enhancing accessibility.
Impact: The 505(b)(2) pathway enabled rapid approval, and the new formulation’s patents provided IP protection, demonstrating how 505(b)(2) can create market-differentiating products [7].
Zembrace SymTouch (Sumatriptan Injection)
Overview: Approved in 2016, Zembrace SymTouch is a prefilled, ready-to-use sumatriptan injection for migraines, developed via 505(b)(2).
Innovation: The prefilled device improved patient convenience over existing sumatriptan formulations, earning patents for the delivery system.
Impact: By leveraging existing sumatriptan data, the approval process was streamlined, and the new IP extended market protection, showcasing 505(b)(2)’s ability to enhance existing therapies [8].
Strategic Considerations for Using 505(b)(2)
Identifying Opportunities
To maximize the 505(b)(2) pathway’s potential, companies should:
- Analyze Market Needs: Identify unmet needs, such as improved delivery or new indications, using market research and tools like DrugPatentWatch to assess patent landscapes [5].
- Evaluate Existing Data: Determine available data (e.g., published literature, FDA findings) to reduce study requirements.
- Assess IP Opportunities: Identify patentable innovations and ensure non-infringement of existing patents.
Regulatory Requirements
While 505(b)(2) reduces study needs, applicants must provide:
- Bridging Studies: To link the new product to the RLD, often involving pharmacokinetic (PK) or pharmacodynamic (PD) studies [9].
- Clinical Trials: For significant changes, such as new indications, requiring new efficacy data.
- CMC Data: Chemistry, manufacturing, and controls information to ensure product quality.
Collaboration with regulatory experts, like those at BioPharma Services, can streamline this process [1].
Intellectual Property Management
Effective IP management involves:
- Filing New Patents: For innovations like formulations or delivery systems.
- Monitoring Competitor Patents: Using DrugPatentWatch to track RLD patents and avoid infringement [5].
- Patent Certifications: Addressing Orange Book patents, potentially challenging invalid or non-infringed patents, which may lead to a 30-month stay if litigation occurs [4].
Commercialization Strategies
Successful commercialization requires:
- Market Differentiation: Highlighting advantages (e.g., ease of use, better efficacy) to physicians and patients.
- Pricing Strategies: Balancing cost with value to ensure payer reimbursement.
- Education Campaigns: Informing stakeholders about the product’s benefits, as seen with Narcan’s public health campaigns.
Challenges and Pitfalls
Regulatory Hurdles
- Data Sufficiency: The FDA may reject relied-upon data if it’s outdated or insufficient, requiring additional studies.
- Study Requirements: Unexpected demands for clinical trials can increase costs and timelines.
Patent Litigation Risks
- Infringement Claims: If the new product is deemed too similar to the RLD, patent holders may sue, potentially delaying approval with a 30-month stay [4].
- Patent Challenges: Competitors may contest new patents, requiring robust IP defense.
Market Acceptance
- Competition: Generics or other 505(b)(2) products may compete, especially post-exclusivity.
- Payer Reimbursement: Insurers may resist covering higher-priced 505(b)(2) products unless clear benefits are demonstrated.
Conclusion
The 505(b)(2) pathway is a powerful tool for pharmaceutical companies seeking to innovate beyond existing drug patents. By leveraging existing data, companies can develop new formulations, indications, or combinations, securing new patents and market exclusivity while reducing development costs and timelines. Case studies like Emflaza, Narcan, and Zembrace SymTouch illustrate the pathway’s potential to address unmet needs and extend product lifecycles. However, success requires strategic planning, robust IP management, and careful navigation of regulatory and market challenges. For business professionals, understanding and leveraging 505(b)(2) can transform patent data into a competitive advantage, driving innovation and profitability in a dynamic industry.
Key Takeaways
- Streamlined Development: The 505(b)(2) pathway reduces development time and cost by leveraging existing data, with 60% of recent NDAs using this route [1].
- Innovation Opportunities: New formulations, indications, and combinations can lead to new patents and exclusivity, extending market life.
- Strategic Planning: Success requires market analysis, regulatory compliance, and IP management, with tools like DrugPatentWatch aiding patent navigation [5].
- Real-World Success: Drugs like Emflaza, Narcan, and Zembrace SymTouch demonstrate the pathway’s ability to deliver innovative therapies.
- Challenges to Address: Regulatory hurdles, patent litigation, and market acceptance must be managed to maximize benefits.
FAQ
- What is the difference between 505(b)(2) and ANDA?
- A 505(b)(2) NDA is for drugs that differ from an RLD but rely on its data, enabling innovation, while an ANDA (505(j)) is for generics requiring bioequivalence and “sameness” to the RLD.
- Can 505(b)(2) be used for biologics?
- No, 505(b)(2) is for small molecule drugs. Biologics follow the Biologics License Application (BLA) pathway [3].
- How long does the 505(b)(2) process take?
- Typically 2–5 years, significantly less than the 10–15 years for 505(b)(1), with average FDA review times of 10 months [1].
- What types of exclusivity can 505(b)(2) products receive?
- They may receive 3-year exclusivity for new clinical investigations, 5-year for NCEs, 7-year for orphan drugs, or 6-month pediatric extensions [3].
- Is it possible to get a patent for a 505(b)(2) product?
- Yes, new formulations, delivery systems, or methods of use can be patented, providing additional IP protection [4].
References
- BioPharma Services. (2022). When is 505(b)(2) a Good Choice for Your New Drug Application? https://www.biopharmaservices.com/blog/phase-1-when-is-505b2-a-good-choice-for-your-new-drug-application/
- Allucent. (2021). Benefits of The 505(b)(2) Pathway For Prodrugs. https://www.allucent.com/resources/blog/benefits-utilizing-505b2-pathway-prodrugs
- FDA. (2021). Applications Covered by Section 505(b)(2). https://www.fda.gov/regulatory-information/search-fda-guidance-documents/applications-covered-section-505b2
- Federal Register. (2016). Abbreviated New Drug Applications and 505(b)(2) Applications. https://www.federalregister.gov/documents/2016/10/06/2016-22690/abbreviated-new-drug-applications-and-505b2-applications
- DrugPatentWatch. (2024). Leveraging 505(b)(2) to Innovate Beyond Existing Drug Patents. https://www.drugpatentwatch.com/blog/leveraging-505b2-to-innovate-beyond-existing-drug-patents/
- DrugPatentWatch. (n.d.). EMFLAZA Loss of Exclusivity (LOE). https://www.drugpatentwatch.com/p/tradename/EMFLAZA
- RHO World. (n.d.). 505(b)(2) Regulatory Pathway: What Are the Advantages and Does Your Product Qualify? https://www.rhoworld.com/505b2-regulatory-pathway-what-are-the-advantages-and-does-your-product-qualify/
- FDA. (2019). Drug Trials Snapshots: EMFLAZA. https://www.fda.gov/drugs/drug-approvals-and-databases/drug-trials-snapshots-emflaza
- The FDA Group. (2024). FDA’s 505(b)(2) Explained: A Guide to New Drug Applications. https://www.thefdagroup.com/blog/505b2