Introduction: The Strategic Imperative of CDMO Partnerships in Modern Pharma
In the dynamic and fiercely competitive landscape of the pharmaceutical and biotechnology industries, the journey from scientific discovery to a marketable drug is fraught with complexities, immense costs, and significant risks. For business professionals at the helm, the strategic decisions made at each turn can dictate the very trajectory of their innovations. One such pivotal decision, increasingly critical in our modern era, revolves around the selection of a Contract Development and Manufacturing Organization (CDMO). But what exactly is a CDMO, and why has its role transcended mere outsourcing to become a strategic imperative for market domination?
What is a CDMO and Why Are They Revolutionizing the Pharmaceutical Industry?
A CDMO, or Contract Development and Manufacturing Organization, is a specialized company that offers comprehensive services spanning the entire drug lifecycle, from initial drug development through to commercial manufacturing for pharmaceutical and biotech firms.1 Unlike a Contract Manufacturing Organization (CMO), which traditionally focuses solely on large-scale production, or a Contract Research Organization (CRO), which primarily handles research and clinical trials, a CDMO integrates both development and manufacturing capabilities under one roof.1 This holistic approach is revolutionizing the pharma industry by streamlining processes, enabling faster, more efficient, and cost-effective production of pharmaceutical products.1
CDMOs bring a wealth of technical expertise in critical areas such as drug formulation, analytical development, regulatory support, and quality control, collaborating closely with clients to bring their pharmaceutical products to market.1 They employ well-qualified chemists, engineers, development specialists, and experienced researchers to manage the entire process from start to finish.1 This comprehensive offering helps pharmaceutical companies overcome significant challenges like cost management, capacity constraints, regulatory compliance, technology adoption, and speed to market.1
The market for these integrated services is expanding at an impressive rate. The global CDMO market was valued at USD 238.92 billion in 2024 and is projected to grow to USD 465.24 billion by 2032, exhibiting a Compound Annual Growth Rate (CAGR) of 9.0% during this forecast period.6 North America, for instance, held a significant market share of 38.59% in 2024.6 This robust growth underscores the increasing reliance of pharmaceutical and biotech companies, particularly small and mid-sized firms with limited in-house capabilities, on CDMOs to reduce operational and capital expenses.6
The distinction between CMO, CRO, and CDMO is crucial for understanding the integrated value these organizations provide. A CDMO’s ability to offer end-to-end solutions, from drug development to commercial manufacturing, signifies a profound shift in the outsourcing paradigm. This integrated approach allows for seamless transitions between development phases and manufacturing, reducing hand-off delays and ensuring greater coordination.7 This means that CDMOs are no longer just a convenient option for delegating tasks; they are a critical enabler for companies to bring complex, novel therapies to market efficiently. Their comprehensive capabilities position them as central to a company’s core strategy, rather than just its operational arm. This evolution from mere outsourced service providers to indispensable strategic partners fundamentally reshapes the drug development and manufacturing landscape.
The Evolving Landscape: From Transactional to Strategic Partnerships
Historically, many pharmaceutical companies viewed outsourcing as a transactional arrangement, a means to offload specific tasks like large-scale manufacturing to reduce costs. However, the pharmaceutical landscape is undergoing a profound transformation. The rise of complex modalities such as cell and gene therapies, antibody-drug conjugates (ADCs), and personalized medicine is reshaping the entire ecosystem.8 These innovations demand deeper technical capabilities, specialized equipment, and intricate regulatory navigation, pushing CDMOs beyond their traditional manufacturing roles.8
Today, CDMOs are increasingly seen as critical innovation partners, supporting everything from early-stage design to commercial-scale production.8 This shift is driven by the sheer complexity and escalating costs of bringing new drugs to market. With an average cost estimated to be over $2.5 billion to develop and launch a new drug 5, and an overall probability of success for new molecular entities from Phase I to approval being only 12% 10, pharmaceutical companies, especially smaller biotechs with limited in-house infrastructure, are compelled to seek comprehensive external support.6
This evolving relationship demands a higher level of collaboration, transparency, and trust.12 As one industry expert noted,
“You shouldn’t view your CDMO as simply a vendor but, rather, as a strategic partner. In turn, the CDMO should view your success as their success, and do what’s necessary to make your firm successful.”
— 12
This mutual investment fosters a “win-win” relationship, essential for navigating the inherent uncertainties of drug development.12 The increasing complexity of drug modalities and the inherent high-risk, high-cost nature of drug development have fundamentally shifted the CDMO-client relationship from a transactional vendor model to a deeply integrated, strategic partnership. This transition is not merely a change in terminology; it reflects a fundamental re-evaluation of how pharmaceutical innovation is achieved. The staggering financial investment and low success rates mean companies cannot afford missteps, making partners who can actively de-risk the process and accelerate timelines incredibly valuable. This leads to a collaborative dynamic where shared success and deep collaboration become paramount, transforming the CDMO into an extension of the client’s own R&D and manufacturing capabilities.
Why Choosing the Right CDMO is Your Next Competitive Advantage
In a market where innovation is currency and speed is paramount, the choice of a CDMO isn’t merely an operational decision; it’s a strategic maneuver that can define a company’s competitive edge and accelerate its path to market domination. Think of it like this: in a high-stakes chess match, a CDMO isn’t just a pawn; it’s a queen, capable of moving across the board to unlock opportunities and defend against threats.
A well-chosen CDMO can significantly accelerate time-to-market, a critical differentiator in a crowded industry.4 They offer access to specialized expertise and state-of-the-art infrastructure that would be prohibitively expensive or time-consuming to build in-house.4 This allows internal teams to focus on core competencies like research and development, rather than getting bogged down in manufacturing logistics or regulatory complexities.4
Moreover, the right CDMO acts as a powerful risk mitigation partner, helping navigate the treacherous waters of regulatory compliance, supply chain vulnerabilities, and technical challenges.4 By leveraging their experience and established quality systems, companies can reduce the likelihood of costly delays, product recalls, or regulatory setbacks.4 Ultimately, this strategic partnership enhances agility, improves operational efficiency, and positions a company to capitalize on market opportunities faster and more effectively than competitors. This approach helps business professionals transform data into market domination.
The strategic selection of a CDMO transcends mere operational efficiency, becoming a core component of a company’s competitive strategy. This is because it enables accelerated innovation, optimized resource allocation, and robust risk management, directly contributing to market leadership. By outsourcing non-core activities to experts, a company can free up its own capital and talent to focus on breakthrough R&D, thereby innovating faster. The ability to launch products quickly and reliably, while managing inherent industry risks, allows a company to capture market share and respond to patient needs more effectively than those relying solely on in-house capabilities or less strategic outsourcing partners. This holistic view elevates CDMO selection from a procurement task to a strategic business decision that directly impacts profitability and market position.
Phase One: Defining Your Needs – The Foundation of a Successful Partnership
Before embarking on the complex journey of selecting a CDMO, a pharmaceutical or biotech company must first look inward. This initial phase, often overlooked in the rush to market, is perhaps the most critical. It’s about conducting a candid self-assessment to clearly define a project’s unique needs, understand internal capabilities, and articulate precisely what is sought from an external partner. Think of it as mapping out a destination before choosing a vehicle; without a clear map, even the most advanced vehicle won’t get there efficiently.
Understanding Your Project’s Development Stage and Specific Requirements
The “right” CDMO is not a universal entity; it’s highly specific to a project’s current development stage and its unique requirements. Is the project at the nascent discovery phase, needing extensive pre-formulation and analytical development support? Or is the molecule in late-stage clinical trials, demanding robust commercial-scale manufacturing capabilities and regulatory submission expertise? It’s crucial to determine whether a CDMO is needed for the entire journey from discovery to commercialization, or if engagement will begin at a specific point, such as preclinical development or clinical research.28
Different stages demand different strengths from a CDMO. An early-phase project might prioritize a CDMO’s flexibility and nimbleness to quickly enter Phase I trials, even if it means potentially switching partners later.29 Conversely, a late-stage program requires a CDMO with proven commercial scale-up capabilities and a seamless transition plan.27 A mismatch between specific needs and a CDMO’s capabilities can result in significant cost overruns, delays, and even regulatory setbacks.27 Therefore, a detailed understanding of a project’s current phase, its projected trajectory, and the precise services required is non-negotiable.27
The effectiveness of CDMO selection hinges on the client’s granular understanding of their own project’s current and future needs, implying that a “one-size-fits-all” CDMO approach is inherently flawed. This goes beyond simply identifying the immediate service needs. It necessitates a foresight into the entire anticipated lifecycle of the drug, from initial research through to commercial production. For example, a small biotech company might initially need only clinical trial material, but if the drug progresses, it will eventually require large-scale commercial manufacturing. Choosing a CDMO that can only handle the early phases might mean a costly and time-consuming technology transfer to another CDMO later on. This highlights that strategic planning involves either selecting a full-service CDMO capable of supporting the entire journey or consciously planning for multiple specialized partners, each choice carrying its own set of trade-offs in terms of cost, time, and risk.
Assessing Molecule Complexity and Therapeutic Area Specialization
Not all molecules are created equal, and neither are all CDMOs. The inherent complexity of a drug candidate—whether it’s a small molecule, a large biologic, a cutting-edge cell or gene therapy, or a highly potent active pharmaceutical ingredient (HPAPI)—will profoundly influence the CDMO choice.8 For instance, novel and emerging biotherapeutics, particularly cell and gene therapies, often require highly specialized equipment and pose unique challenges, such as stringent chain of custody and identity requirements for living materials.31
A CDMO’s “legacy in monoclonal antibody (mAb) process development and manufacturing is no substitute for specific and relevant experience in your novel molecule”.31 This is a critical distinction. While a CDMO might boast decades of experience, if that experience is predominantly in traditional small molecules and a project involves, say, an mRNA therapeutic, their general track record may not translate directly to success. A partner is needed with a proven track record in the specific drug type or therapeutic area, ideally with case studies or references related to similar projects.26
CDMOs specializing in niche forms, like nasal sprays or high-potency compounds, bring invaluable experience in developing robust manufacturing processes and ensuring compliance with stringent standards for these unique products.5 This specialized expertise ensures that the CDMO can effectively manage the unique challenges a project presents, from formulation development to regulatory approval.8 The implication here is that “experience” in CDMO selection is not a generic metric of longevity but a highly contextual and modality-specific attribute. Its value lies in its direct applicability to the client’s unique drug type and therapeutic area. This means that merely looking at a CDMO’s overall years in business or broad client list is insufficient. Instead, a thorough due diligence process must involve asking for specific case studies and technical details on projects involving molecules with similar complexities, delivery systems, or therapeutic targets. This granular assessment ensures that the chosen CDMO possesses the precise, relevant expertise needed to navigate the unique scientific and technical hurdles of the client’s particular drug development journey, significantly increasing the probability of success.
Internal Capabilities vs. Outsourcing Needs: A Candid Self-Assessment
Before even beginning to scout for external partners, a fundamental question must be addressed: what are internal capabilities, and where are the gaps? This isn’t just about identifying what cannot be done, but also what should not be done in-house. Developing and manufacturing pharmaceutical products can be incredibly expensive, requiring substantial upfront investments in specialized equipment and facilities.1 For many companies, particularly small to mid-sized biotechs, allocating extensive capital towards building and maintaining in-house infrastructure is simply not feasible, or it diverts crucial funds from core research and development activities.5
This is where the strategic value of a CDMO truly shines. Outsourcing allows access to cutting-edge technology and resources without the significant time and money investment required for in-house facilities.20 It enables companies to “delay any CAPEX investments” and leverage the CDMO’s existing infrastructure, mitigating financial risks associated with uncertain drug development outcomes.5 Moreover, it provides access to a pool of qualified chemists, engineers, and research specialists, bypassing the resource-intensive aspects of recruiting and retaining such highly specialized staff.5
However, outsourcing also means relinquishing some control.31 Companies must weigh the financial savings and access to expertise against the potential loss of direct oversight. A thorough internal assessment should identify not only the technical and capacity gaps but also the strategic areas where outsourcing provides the greatest leverage, allowing a company to focus on its unique strengths and accelerate its core mission.4 The decision to outsource to a CDMO is a strategic financial and operational calculus. It allows companies to convert significant capital expenditure (CAPEX) into operational expenditure (OPEX) while gaining access to specialized, otherwise unattainable, capabilities. This is not merely a cost-saving measure on a single project, but a fundamental shift in financial strategy. By avoiding the massive upfront costs of building and maintaining facilities, which can run into billions of dollars, companies can reallocate their capital to high-value activities like innovative R&D, clinical trials, or market expansion. This approach provides greater financial flexibility and significantly reduces the financial risk associated with the uncertain outcomes inherent in drug development. It’s about optimizing the return on investment by focusing internal resources where they can generate the most unique value, while leveraging external partners for capital-intensive or highly specialized functions.
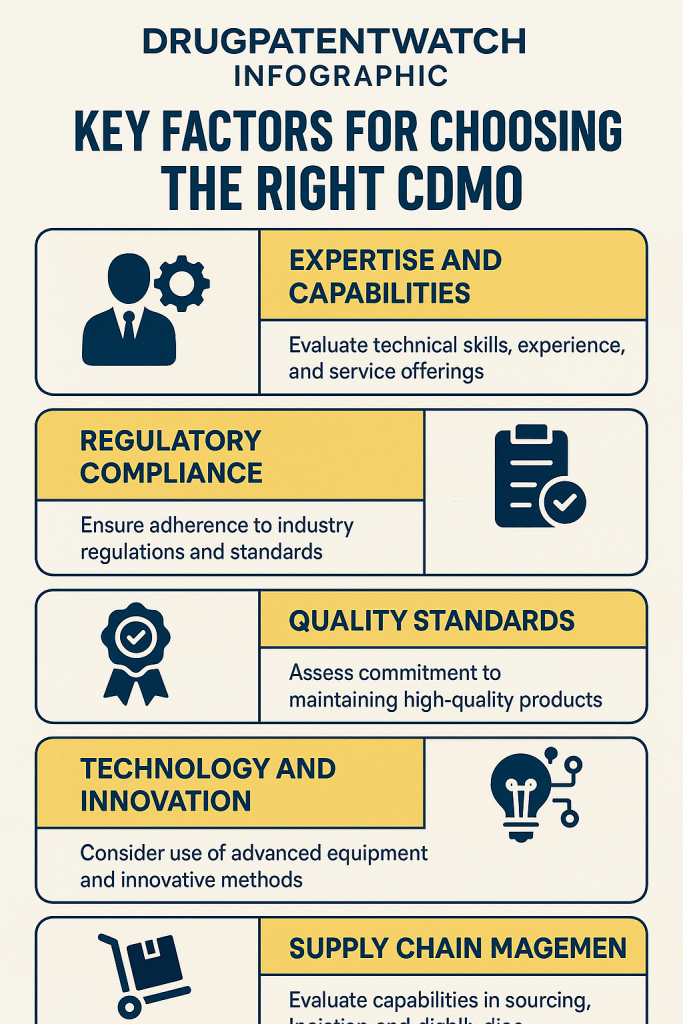
Phase Two: Evaluating Potential Partners – The Core Pillars of CDMO Excellence
Once a crystal-clear understanding of internal needs and project requirements is established, the real work of evaluating potential CDMO partners begins. This phase is akin to meticulously inspecting the foundational pillars of a skyscraper; each must be robust, perfectly aligned, and built to withstand immense pressure. For CDMOs, these pillars are Quality, Expertise, Technology, Capacity, Communication, Financial Stability, Supply Chain Resilience, and Intellectual Property Protection. Neglecting any one of these can lead to catastrophic structural failures in a drug development program.
Unwavering Commitment to Quality and Regulatory Compliance
In pharmaceutical manufacturing, quality is not merely a desirable trait; it is the absolute bedrock upon which patient safety and market success are built. There is no room for compromise. A chosen CDMO must demonstrate an unwavering commitment to quality and a flawless track record in regulatory compliance.
Building Blocks of Excellence: Robust Quality Management Systems (QMS) and GMP Adherence
A CDMO’s quality management system (QMS) serves as the backbone of its operations, ensuring that products are consistently produced and controlled according to stringent quality standards.26 This includes adherence to current Good Manufacturing Practices (cGMP) requirements, which are non-negotiable.12 Look for a CDMO that can demonstrate validated analytical methods, strong documentation practices, and robust systems for investigating and addressing any deviations.34 Their quality systems should guarantee consistent manufacture of a product to specification, batch after batch.34
Regular inspections by regulatory agencies like the FDA (U.S. Food and Drug Administration) and EMA (European Medicines Agency) are a given, and a quality CDMO should successfully pass these audits consistently.27 They should also proactively employ risk-based approaches and Process Analytical Technology (PAT) to optimize process parameters, enhance product consistency, and reduce risk at final release testing.24
While GMP adherence and a robust QMS are fundamental, true quality excellence in a CDMO is characterized by a proactive, integrated approach to Quality by Design (QbD) and continuous improvement, moving beyond mere compliance checklists. This means that a CDMO doesn’t just react to quality issues but actively designs processes to prevent them. For example, implementing QbD principles means understanding the critical quality attributes of a drug product from the outset and designing manufacturing processes to consistently achieve them, rather than simply testing for quality at the end.37 This proactive stance, leveraging tools like PAT for real-time monitoring and control, indicates a more resilient and reliable partner. It’s the difference between a company that merely avoids regulatory penalties and one that genuinely strives for optimal product integrity and patient safety.
Decoding Regulatory Signals: Interpreting FDA 483s and Warning Letters for Due Diligence
The regulatory landscape is a minefield, and a CDMO’s history with agencies like the FDA and EMA offers invaluable clues about their operational integrity. During due diligence, it is imperative to delve into their compliance history, specifically looking at FDA Form 483 observations and Warning Letters.
A Form 483 is issued at the conclusion of an FDA inspection when investigators observe conditions that may constitute violations of the Food, Drug, and Cosmetic (FD&C) Act.40 It serves as a guide for voluntary corrective action, requiring a company response within 15 working days.41 Critically, a 483 is
not a final determination of non-compliance.41
A Warning Letter, however, is a much more serious matter. It is a public document that explicitly states the exact regulation a company is in violation of, typically sent when issues from a Form 483 were not fixed properly or if the violations are severe.41 A Warning Letter signals that the FDA believes the company is breaking important rules and can impact requests for licenses or applications, potentially leading to legal action if not addressed.41 Common reasons for receiving these include data integrity issues, inadequate investigations, poor documentation practices, and substandard equipment or facilities.42
When evaluating a CDMO, investigate their compliance history to ensure they meet stringent quality management systems.27 Transparency in how they manage inspections and audits is key.27 Any warning letters or compliance issues should be highlighted and thoroughly considered, as they can indicate systemic problems that could lead to costly delays, product recalls, or damaged reputations for a product.25 FDA 483s and Warning Letters serve as critical diagnostic tools, revealing not just isolated compliance failures but deeper systemic issues within a CDMO’s operational maturity, quality culture, and risk management framework. The progression from a 483 to a Warning Letter often indicates a failure to adequately address observations, suggesting a reactive rather than proactive approach to quality. For instance, repeated 483s for “inadequate investigations” or “poor documentation practices” point to fundamental weaknesses in the CDMO’s quality culture and their ability to identify and correct root causes.42 This means that a client’s due diligence should extend beyond a simple check for regulatory flags; it requires a deep dive into the nature of the observations, the CDMO’s response, and the effectiveness of their remediation efforts. A CDMO that consistently receives and effectively resolves minor observations demonstrates a robust QMS, while one with recurring or escalating issues may pose significant risks to a client’s project timelines, budget, and market reputation.
Beyond Compliance: Indicators of a True Quality Culture
While regulatory compliance is essential, a truly excellent CDMO goes beyond merely ticking boxes. They cultivate a deep-seated “quality culture” – a mindset where every employee, from the lab bench to senior leadership, is committed to doing the right thing at the right moment, prioritizing quality and patient impact.14
As Liesbeth Foesters from UCB succinctly put it,
“While we, as an industry, are all aligned that we want the right quality product, for CDMOs it not always the first priority. Well, for us, it is.” She emphasized that true quality “is about making sure you’re doing the right thing at the right moment. You think efficiency, but you also think business, making sure you’re doing it right is super important.”
— 14
This cultural emphasis is echoed by Stefan Bouckaert from J&J, who noted, “What is for me very essential is a culture… you sense it really working with some of the companies. It can be very small CDMOs that you see have a good culture, and a willingness to really build something”.14
Indicators of such a culture include:
- Transparency and Trust: A CDMO that is transparent about its processes, data, and even challenges, builds trust. “The more transparent you are with your customer, the less they are going to worry. The less you are transparent, the more scrutiny you are going to create,” as Mabonzo explained.14 This open dialogue is crucial for effective problem-solving and risk management.12
- Employee Empowerment and Training: A strong quality culture fosters respect and empowers employees to identify issues and drive continuous improvement.14 This requires ongoing training and development programs to enhance their skills and knowledge.37
- Proactive Problem Solving: Rather than just reacting to deviations, a quality-driven CDMO will have robust systems for root-cause analysis and preventive actions, ensuring issues don’t recur.35
- Critical Thinking and Soft Skills: Beyond technical knowledge, quality professionals need strong soft skills to influence effectively and lead conversations.14 This holistic approach to quality is what truly safeguards a product and reputation.
A CDMO’s quality culture, characterized by proactive transparency, empowered personnel, and a commitment to continuous improvement, is a superior predictor of long-term partnership success and risk mitigation than mere regulatory compliance. Compliance is a baseline, a minimum requirement, but a true quality culture is an aspirational state where quality is embedded in every decision and action, even when no one is watching.39 This means that while audit reports are necessary, they are insufficient for a complete assessment. Clients must conduct site visits, observe daily operations, and engage with personnel at various levels to gauge the true cultural commitment to quality. A CDMO that fosters transparency, empowers its employees to speak up about issues, and invests in their continuous development is far more likely to anticipate and resolve problems before they escalate. This deeper cultural alignment ensures that when inevitable challenges arise, both parties will work collaboratively to find solutions rather than engaging in blame, thereby preserving timelines, budgets, and ultimately, patient safety.
Deep Expertise and Proven Track Record
Choosing a CDMO is like selecting a seasoned guide for a treacherous expedition; a company needs someone who has not only traversed similar terrain but has done so successfully, time and again. Their expertise and track record are invaluable assets that can significantly de-risk a project.
Experience That Matters: Relevant Background Across Modalities and Dosage Forms
A CDMO’s experience is a critical indicator of its capability to ensure project success.24 However, it’s vital to look beyond the number of years in operation and focus on the
relevance of their experience to a specific project. Not every CDMO will have equal experience across every dosage form, molecule type, or therapeutic area.27 For instance, a CDMO with a long history in small molecule manufacturing might not possess the specialized expertise required for complex biologics, cell and gene therapies, or high-potency active pharmaceutical ingredients (HPAPIs).8
A thorough exploration of a potential CDMO’s portfolio and a search for case studies or references directly related to the type of drug intended for development are essential.27 If a CDMO has successfully navigated projects involving similar drugs, it is far more likely to effectively manage a project’s unique challenges.27 This varied experience, encompassing a range of therapeutic modalities, delivery systems, packaging options, and scales, can be invaluable, especially for companies that lack the financial resources to accommodate rework or delays.24
“Experience” in CDMO selection is not a generic metric of longevity but a highly contextual and modality-specific attribute, where relevance to the client’s unique drug type and therapeutic area is paramount for successful project execution. This means that a CDMO’s general reputation, no matter how stellar, is insufficient if it doesn’t directly align with the specific technical demands of the client’s drug. For example, a CDMO renowned for its expertise in oral solid dosages might lack the specialized containment facilities and analytical capabilities needed for a highly potent oncology drug. Therefore, clients must perform granular due diligence, asking for specific case studies and technical details on projects involving similar molecules or therapeutic areas. This level of scrutiny ensures that the chosen CDMO possesses the precise, relevant expertise needed to navigate the unique scientific and technical hurdles of the client’s particular drug development journey, significantly increasing the probability of success and mitigating the risk of costly reworks.
Learning from Success: The Value of Case Studies and Client References
Beyond a CDMO’s self-proclaimed expertise, their actual track record speaks volumes. A critical part of due diligence should involve requesting and scrutinizing case studies and, crucially, speaking directly with their past and current clients.27 This peer-to-peer perspective offers invaluable, unfiltered information about their working style, adherence to quality standards, and how they navigate challenges.28
Ask for examples of similar projects they’ve undertaken and how they managed unexpected issues or delays.33 A reputable CDMO should be willing to share such information and connect with references who can attest to their capabilities, flexibility, and communication effectiveness.27 This direct feedback can help gauge their willingness to adapt to a client’s quality management systems, work with various teams, and communicate according to specific needs – all core to achieving a flexible and successful partnership.24 Remember, unforeseen challenges are inevitable in drug development; the mark of a seasoned CDMO is its ability to quickly overcome problems and avoid significant delays.33 Client references and detailed case studies provide empirical validation of a CDMO’s claimed expertise and operational approach, offering critical insights into their problem-solving capabilities and collaborative fit. This goes beyond the glossy brochures and sales pitches, providing a real-world view of how the CDMO operates under pressure. For instance, a case study might detail how a CDMO successfully scaled up production for a complex biologic despite unforeseen raw material shortages, showcasing their supply chain resilience and problem-solving acumen. Speaking directly with former clients can reveal whether the CDMO is truly a partner in problem-solving or merely a service provider that adheres strictly to the contract, regardless of evolving project needs. This level of due diligence allows clients to uncover not just successes, but also how challenges were handled, providing a realistic picture of what a partnership might entail and whether the CDMO’s operational philosophy aligns with their own.
Cutting-Edge Technological Capabilities and Innovation
In an industry driven by scientific breakthroughs, a CDMO’s technological prowess is no longer a luxury but a fundamental necessity. The ability to leverage advanced technologies and embrace innovation can dramatically impact the efficiency, speed, and quality of drug development and manufacturing processes.
Embracing the Future: Continuous Manufacturing and 3D Printing in Pharma
The pharmaceutical industry is rapidly adopting advanced manufacturing technologies, and leading CDMOs are at the forefront of this transformation. Two prominent examples are continuous manufacturing and 3D printing.
Continuous Manufacturing (CM), or flow chemistry, is revolutionizing drug substance manufacturing by offering significant advantages over traditional batch processes.48 These include:
- Increased Efficiency and Speed: CM allows for uninterrupted production, significantly boosting output and reducing the time for large-scale manufacturing. It can shorten production times from weeks to a single day.4
- Reduced Waste and Environmental Impact: CM processes can minimize hazardous reagents and solvents, leading to a safer and more environmentally sustainable manufacturing process.48
- Improved Product Quality and Consistency: Real-time monitoring and control of critical process parameters in CM lead to greater understanding of the underlying chemistry, allowing for more precise control and fewer variations in the final product.48
- Faster Process Development and Scale-Up: CM can significantly reduce the time and resources needed for process development and scale-up, enabling faster market entry.48
3D Drug Printing (Additive Manufacturing) is another transformative technology, enabling the production of customized medications with unparalleled flexibility.50 Its benefits include:
- Precision Dosing and Complex Geometries: It allows precise control over drug dosage, shape, size, and release profiles, facilitating personalized treatments and creating complex drug geometries that traditional methods cannot achieve.50
- Rapid Prototyping and Formulation Optimization: Valuable during early R&D, 3D printing accelerates the creation and evaluation of drug prototypes, significantly streamlining formulation development.52
- Reduced API Consumption: This technology can enable the consumption of less Active Pharmaceutical Ingredient (API) for initial formulation trials, leading to cost savings and improved sustainability.52
CDMOs are actively investing in these technologies to meet client demand and boost their competitive edge.4 Partnering with a CDMO that embraces such innovations can lead to faster production cycles, reduced waste, improved scalability, and enhanced compliance for products.53 CDMOs that proactively invest in and integrate advanced manufacturing technologies like continuous manufacturing and 3D printing are not merely keeping pace; they are actively shaping the future of pharmaceutical production. This offers clients a pathway to unprecedented speed, customization, and sustainability. These technologies represent a paradigm shift from traditional batch processes, enabling drug developers to achieve efficiencies and product characteristics previously unattainable. For instance, continuous manufacturing allows for uninterrupted production, drastically cutting down lead times and enabling real-time quality control, which translates directly to faster market entry and a more consistent product.48 Similarly, 3D printing opens doors for personalized medicine, allowing for precise dosage adjustments and complex drug release profiles tailored to individual patient needs.51 Choosing a CDMO with these capabilities means investing in a future-proof manufacturing process that can deliver novel therapies faster, more precisely, and more sustainably, directly impacting market position and patient outcomes.
The Intelligent Edge: Leveraging AI and Automation for Optimized Processes
Beyond physical manufacturing advancements, the digital revolution, particularly in Artificial Intelligence (AI) and Machine Learning (ML), is profoundly impacting CDMO operations. AI is no longer a futuristic concept but a tangible tool for optimizing drug development and manufacturing processes.
CDMOs are increasingly leveraging AI and ML to:
- Optimize Drug Formulation and Production Methods: AI can analyze vast amounts of data to predict formulation stability over long-term storage, optimize production processes, and assess how a drug will perform in trials.4 This can dramatically reduce development timelines and costs.50
- Enhance Manufacturing Efficiency and Quality Control: AI-driven process automation boosts throughput and minimizes errors.57 AI agents can monitor production data, detect deviations in real-time, and even self-correct or alert human operators, ensuring consistent, high-quality production.58
- Improve Predictive Analytics and Resource Optimization: AI-based solutions offer predictive capabilities to anticipate events and optimize resource allocation, recommending suitable scenarios for drug development based on available resources, regulatory requirements, and market demand.56 This includes AI-driven forecasting to anticipate demand and optimize resources for CDMOs themselves.59
- Streamline Regulatory Compliance: AI can autonomously analyze regulatory updates, ensuring compliance across jurisdictions and even suggesting modifications in behaviors, processes, or documentation.58
The integration of AI and automation allows CDMOs to transform into “highly adaptive and more, well, intelligent partners”.58 This intelligent edge enables proactive decision-making before issues become costly delays, ultimately enhancing efficiency, quality, and safety across all stages of drug development and production.56 The strategic adoption of AI and automation by CDMOs represents a paradigm shift from reactive problem-solving to proactive, predictive optimization across the entire drug lifecycle. This offers clients unparalleled agility, risk mitigation, and data-driven decision-making capabilities. For example, AI-powered systems can analyze historical manufacturing data to predict potential equipment failures before they occur, enabling proactive maintenance and preventing costly downtime.53 Similarly, AI agents can monitor complex production workflows in real-time, instantly flagging deviations and even suggesting corrective actions, thereby ensuring consistent product quality and reducing the risk of batch failures.58 This translates to significantly de-risked projects, faster identification and resolution of issues, and more reliable timelines for clients. Ultimately, CDMOs leveraging AI offer a fundamentally different level of operational excellence and strategic foresight, allowing clients to “supercharge efficiency” 60 and achieve a competitive advantage through data-driven insights and enhanced agility in a rapidly evolving market.
Scalable Capacity and Operational Flexibility
Imagine a drug suddenly showing blockbuster potential in clinical trials. Can a CDMO ramp up production from clinical batches to commercial scale without missing a beat? Or conversely, if a project faces unforeseen delays, can they adjust without imposing hefty penalties? A CDMO’s capacity and flexibility are crucial for navigating the inherent uncertainties of drug development.
Forecasting Demand: Assessing Current and Future Production Needs
A critical consideration when choosing a CDMO is their ability to meet current production needs and, more importantly, to scale up as a product moves through development stages to full commercial manufacturing.23 This requires a thorough understanding of projected demand, including the number of patients in clinical trials, dosing regimens, and overall process yield.29
However, the reality of the CDMO market, particularly for novel and complex therapies like viral vectors for gene therapies, often presents challenges. There is currently limited CDMO capacity for viral vector manufacturing, leading to significant wait times—sometimes 6 months to 2 years—which can be devastating for a drug program.31 This highlights the importance of inquiring about a potential CDMO’s current workload, availability, and potential bottlenecks to avoid delays.28 A CDMO must have the capacity to produce the amount of product needed and be able to accurately scope the project based on that need.29 Capacity assessment is a dynamic forecasting exercise, demanding that CDMOs demonstrate not just current availability but also a robust, flexible plan for scaling production up (or down) in response to unpredictable clinical and commercial demands. The stark reality of “long wait times” and “limited CDMO capacity for viral vector manufacturing” 31 underscores that simply asking “do you have capacity?” is insufficient. Clients must probe for the CDMO’s
capacity planning methodologies, their ability to handle demand volatility 21, and their
flexibility to adapt to changing volumes.24 A CDMO that can provide a clear capacity forecast and typical lead times, and has a track record of adapting to changing production volumes without compromising quality, is a strategic asset. This proactive approach to capacity planning is vital for mitigating risks and ensuring a smooth transition from clinical trials to commercialization, ultimately impacting market entry and revenue generation.
Navigating Bottlenecks: Strategies for Mitigating Capacity Constraints and Ensuring Agility
Given the industry’s capacity challenges, particularly for advanced therapies, CDMOs are employing various strategies to ensure agility and mitigate bottlenecks. For clients, understanding these strategies is key to selecting a resilient partner.
Key strategies include:
- Leveraging Scalable Technologies: Good CDMOs employ scalable technologies, such as single-use systems in biologics manufacturing, which can streamline processes and offer greater flexibility.22
- Flexible Manufacturing Capabilities: CDMOs should offer capabilities that can be adjusted to meet the unique needs of different projects, whether for niche market drugs or high-volume commercial products.7 This includes configurable production lines that can support scalable operations at every stage of a drug product’s life cycle.24
- Proactive Supply Chain Management: A CDMO should have robust supply chain management, including raw material sourcing and contingency plans, to ensure materials are consistently available and reduce the risk of production delays.1
- Modular Options for In-House Capacity: The rise of modular manufacturing solutions, which can build a full manufacturing facility in 1.5 to 2 years (or 10-12 months with a “box in box” approach), offers companies the option to bring manufacturing in-house if CDMO delays become too significant.31 A forward-thinking CDMO might even support this eventual transition, demonstrating a true partnership approach.
A CDMO’s ability to balance speed and optimized supply chain management, staffing, and process times is as critical as achieving an accelerated commercial launch.24 They should be flexible enough to manage unforeseen issues without major budget implications and adjust costs if the project needs to pivot or expand.27 Beyond simply having capacity, a CDMO’s true agility lies in its proactive strategies for mitigating capacity limitations, such as adopting scalable technologies and diversifying production footprints. These strategies directly impact a client’s ability to respond to market shifts and secure supply. For example, by investing in flexible, multi-product facilities or leveraging single-use bioreactors, a CDMO can quickly pivot between different projects or scale production up or down without significant retooling or downtime.22 This adaptability is crucial in a volatile market where demand can fluctuate rapidly. Furthermore, a CDMO that proactively manages its supply chain through dual-sourcing critical materials or investing in domestic manufacturing capabilities can significantly reduce the risk of disruptions due to geopolitical tensions or raw material shortages.62 This strategic foresight ensures that the client’s drug program won’t be derailed by external capacity issues, providing a more reliable and secure path to market.
Transparent Communication and Robust Project Management
Effective communication is the lifeblood of any successful partnership, and in the complex world of drug development, it’s nothing short of foundational. Without clear, consistent, and transparent communication, even the most promising CDMO partnership can quickly unravel.
The Lifeline of Partnership: Dedicated Project Managers and Clear Communication Protocols
Clear, consistent, and transparent communication is the foundation of a successful CDMO partnership.11 It’s the mechanism that manages expectations, keeps timelines on track, and helps avoid potential setbacks.27
Look for a CDMO that provides:
- Dedicated Project Managers: A single point of contact who serves as the primary liaison, coordinating internal teams and ensuring seamless project execution.27
- Robust Project Monitoring Tools: Tools that provide real-time updates on progress, risks, and upcoming activities.34
- Regular Status Meetings: Consistent weekly or bi-weekly calls, complemented by monthly governance meetings, to discuss progress, address concerns, and make necessary adjustments.34
- Clear Communication Protocols: Defined channels and escalation pathways for urgent updates, such as quality control failures or deviations.25 Real-time communication and notification to the client are required, especially regarding deviations or non-conformances.66 Production should not continue until the issue has been evaluated and resolved.66
This robust communication structure ensures that both parties are aligned on project expectations, timelines, and deliverables, fostering smoother collaboration.61 Communication in CDMO partnerships is not merely about transmitting information but about actively building trust, enabling proactive problem-solving, and ensuring dynamic alignment through a structured, two-way dialogue. The analogy of “money in the bank” for trust 12 highlights that communication is a continuous investment, not a one-off task. A CDMO that provides dedicated project managers and implements robust monitoring tools and regular status meetings demonstrates a commitment to transparency and proactive management. This structured approach allows for early identification of potential issues, preventing small problems from “snowballing into unmanageable crises”.25 It fosters an environment where challenges are quickly surfaced and collaboratively addressed, ensuring that both parties are always in sync and working towards shared objectives, even when unforeseen circumstances arise.
Cultivating Synergy: Fostering a Collaborative Partnership and Cultural Fit
Beyond the technical and contractual aspects, the human element—the “cultural fit”—is a subtle yet powerful determinant of a CDMO partnership’s success. A company’s partnership with a CDMO is a long-term engagement that can span years to decades.27 Therefore, the CDMO should align with a company’s goals, values, and underlying way of working.27
This means fostering a truly collaborative relationship, moving beyond a purely transactional dynamic.11 As industry leaders emphasize, “That relationship is a two-way street, and honesty pays dividends throughout the process”.11 Look for partners who demonstrate:
- Mutual Respect and Trust: A willingness to value each other’s expertise and work collaboratively towards common goals.11
- Open Culture: An environment that encourages open dialogue, even when discussing potential risks or challenges.12
- Adaptability: The ability to adapt their processes to accommodate preferences for project management, communication, and technical approaches.24
- Shared Commitment to Patient Impact: A CDMO that identifies at a personal level with a product and patients, reinforcing a sense of purpose behind the process.31
Building personal relationships with individuals at a CDMO can be invaluable, as problems will inevitably arise, and trust will be crucial in navigating them.12 This synergy ensures that both teams are pulling in the same direction, treating a client’s success as their own.12 Cultural fit and the cultivation of a truly synergistic partnership are critical, often overlooked, determinants of long-term CDMO success. They act as a crucial buffer against unforeseen challenges and foster resilient collaboration. While technical capabilities and cost are obvious factors, the emphasis on “cultural fit” 14 and the “strategic partner” relationship 11 highlights a deeper truth. A CDMO that shares a client’s values and fosters an open, trusting environment is more likely to be proactive in identifying and resolving issues, rather than waiting for problems to escalate. This means that due diligence must extend beyond audits and technical assessments to include evaluating the “rapport” 70 between teams and the CDMO’s willingness to adapt to client systems. When both parties feel a shared sense of purpose and mutual respect, they are better equipped to navigate the inevitable complexities of drug development, ensuring continuity and minimizing disruptions.
Financial Transparency and Stability
The financial health of a CDMO partner is not just their concern; it’s a client’s too. A CDMO’s financial stability directly impacts its ability to deliver services reliably over the long term, invest in necessary technologies, and weather economic fluctuations.
Beyond the Sticker Price: Decoding CDMO Pricing Models and Evaluating Quotes
When a CDMO quote lands in an inbox, resist the urge to simply skim the total. Decoding pricing isn’t just about saving money; it’s about leveraging data to dominate the market.72 CDMOs typically employ various pricing models, and understanding them is crucial:
- Fee-for-Service: In this model, payment is for specific, defined tasks (e.g., formulation development or a clinical batch). It’s ideal for early-stage projects with uncertain timelines, but costs can escalate if the scope creeps.72
- Time and Materials: Billing is based on labor hours and materials used. This offers flexibility for complex projects, but transparency in tracking hours and material costs is key to avoid unexpected hefty tabs.72
- Fixed-Price Contracts: This model locks in costs upfront, providing predictability for budgeting. However, CDMOs often build in buffers for risk, so the price might be higher than with a variable model, akin to buying insurance.72
When evaluating quotes, look beyond the bottom line. Request a line-item breakdown to identify hidden fees or overcharges.72 Compare quotes meticulously, standardizing the evaluation with a checklist, as not all CDMOs bundle services equally.72 Most importantly, assess value, not just cost. A low bid might indicate cut corners or delays, which can cost more in the long run. As Warren Buffett famously said, “Price is what you pay; value is what you get”.72 Focus on the return on investment (ROI) and whether the CDMO can deliver on time and quality.72
The pricing structure of a CDMO is not just a financial detail; it’s a strategic tool that can significantly impact a client’s budget predictability and risk exposure. Understanding the nuances of fee-for-service, time and materials, and fixed-price models is crucial for aligning financial commitments with project uncertainties. For example, while a fixed-price contract offers budget certainty, it might include a premium for the CDMO’s risk absorption. Conversely, a time and materials contract offers flexibility for evolving projects but demands rigorous oversight to prevent cost overruns. The implication is that clients must engage in detailed negotiations, pushing for transparency in cost breakdowns and understanding the rationale behind pricing.72 This allows for a true “apples-to-apples” comparison and ensures that the chosen CDMO’s pricing model supports the client’s financial strategy, rather than creating unforeseen liabilities.
Financial Health and Long-Term Viability: Avoiding Red Flags
The financial stability of a CDMO is paramount for ensuring reliable, long-term service delivery. A CDMO with solid financial backing can invest in state-of-the-art technology, maintain adequate inventory levels, and retain skilled personnel, all crucial for project success.26 Conversely, a CDMO facing financial distress could abruptly halt production, leaving clients scrambling to find new partners and causing significant delays that can affect funding or project timelines.61
Red flags to watch out for include:
- Lack of Credit History or Backing: This can signal instability.61
- Unrealistic Low Bids: If a CDMO’s quote is significantly lower than competitors, it might indicate a lack of understanding of the project’s specifics or a willingness to cut corners, leading to costly delays or quality issues later.11
- Generic Proposals: A CDMO that provides a generic cost sheet rather than a unique proposal tailored to the project’s needs may not have thoroughly assessed the scope, potentially leading to hidden costs.70
A stable financial base is particularly important for long-term projects that require ongoing investment in manufacturing and development.26 Due diligence should include reviewing the CDMO’s financial statements, tax filings, and debt obligations to assess their financial health and ability to sustain operations.73 The financial stability of a CDMO is not merely a background detail; it is a direct determinant of long-term reliability and the continuity of a client’s drug supply. A CDMO’s ability to deliver services consistently over the long term, invest in quality systems, and attract top talent is directly tied to its financial health. This means that a CDMO facing financial distress poses a significant risk of disruptions, including production halts or even closure, which can have devastating consequences for a client’s drug program, leading to costly delays, reputational damage, and lost market opportunities.25 Therefore, rigorous financial due diligence, including scrutinizing their balance sheets and identifying any red flags like hidden costs or unrealistic bids, is essential. This proactive assessment ensures that the chosen CDMO is a robust and dependable partner, capable of supporting the entire lifecycle of a drug without unexpected financial or operational interruptions.
Supply Chain Resilience and Global Reach
In an increasingly interconnected yet volatile world, a CDMO’s supply chain resilience and global reach are not just logistical advantages; they are strategic necessities. The ability to navigate geopolitical shifts, raw material shortages, and fluctuating demand directly impacts a client’s ability to maintain consistent supply and accelerate market entry.
Mitigating Disruptions: Diversification, Dual-Sourcing, and Domestic Manufacturing
The COVID-19 pandemic exposed the fragility of global supply chains, highlighting the critical need for resilience. Leading CDMOs are now actively building more robust and secure supply chains to mitigate disruptions.1 Key strategies include:
- Diversifying Suppliers: Reducing dependency on single-source vendors for raw materials and components. This helps insulate projects from geopolitical risks and lead time variability.59
- Dual-Sourcing: Procuring critical raw materials from two different vendors, often in different geographies, to provide redundancy and minimize the impact of unanticipated supply disruptions.62
- Domestic/Regional Manufacturing: Investing in local or regional manufacturing capabilities to reduce reliance on distant supply chains, minimize risks from tariffs, and streamline logistics.62 This trend, often termed “reshoring,” is gaining momentum, especially with policies like the BioSecure Act influencing supply chain strategies.8
A CDMO that can manufacture key starting materials (KSMs) in-house or maintain stock of essential reagents in controlled storage facilities further enhances supply chain security, reducing dependency on just-in-time sourcing.63 These proactive measures ensure consistent supply and help turn tariff-related complexities into competitive advantages.62 A CDMO’s true agility lies in its proactive strategies for mitigating capacity limitations, such as adopting scalable technologies and diversifying production footprints. These strategies directly impact a client’s ability to respond to market shifts and secure supply. The shift away from “just-in-time” operations, driven by global disruptions and geopolitical tensions, means that CDMOs must build redundant and flexible supply chains.62 This involves not just diversifying suppliers but also strategically dual-sourcing critical materials from different regions and investing in domestic manufacturing capabilities. For clients, this translates to a significantly reduced risk of production delays, raw material shortages, and increased costs due to external factors. A CDMO that demonstrates robust supply chain engineering and proactive risk management is not just a service provider; it’s a strategic partner that safeguards a client’s market access and ensures the continuity of life-saving therapies.
Global Footprint and Regional Nuances: Impact on Logistics and Regulatory Compliance
In today’s globalized pharmaceutical market, a CDMO’s geographical footprint and understanding of regional nuances are increasingly important. The location of a CDMO can significantly affect logistics, project management, and regulatory compliance.26
Considerations include:
- Proximity to Client Operations: Closer proximity minimizes travel time for client staff and helps ensure a smoother technical transfer process.31
- Understanding Regional Regulations: A good CDMO should understand specific regional regulations where a product will be marketed (e.g., FDA in the US, EMA in Europe) and be able to manage cross-border supply chains without delays.26
- Consistency Across Facilities: For companies pursuing distributed manufacturing, it’s crucial that a global CDMO maintains consistency in procedures, equipment, automation, and consumables across its various facilities if site-to-site technology transfer is desired.31
While North America currently dominates the CDMO market, with a 38.59% share in 2024 and projected growth to USD 275.6 billion by 2032 6, regions like Asia-Pacific (particularly Japan and China) are experiencing rapid growth, offering cost advantages.72 However, these cost benefits must be weighed against potential regulatory complexities, intellectual property risks, and supply chain vulnerabilities associated with different regions. A CDMO with robust global capabilities can streamline issues such as shipping times, customs clearance, and differences in regulatory jurisdictions, thereby accelerating market entry.27 A CDMO’s global reach, coupled with its understanding of regional nuances, is not just a logistical convenience but a strategic imperative that directly impacts market access and regulatory efficiency. The ability to navigate diverse regulatory environments, manage complex cross-border supply chains, and adapt to regional market demands is crucial for global commercialization. For instance, a CDMO with facilities in both the US and Europe can streamline regulatory submissions for both FDA and EMA approvals, reducing duplication of effort and accelerating market entry into key territories. Furthermore, understanding regional supply chain dynamics and potential trade barriers is vital for ensuring uninterrupted supply. This means that a CDMO’s global footprint must be supported by deep local expertise and consistent quality systems across all its sites, ensuring that a client’s product meets all necessary standards regardless of its final market destination.
Intellectual Property Protection
In the pharmaceutical and biotechnology industries, intellectual property (IP) is the lifeblood of innovation. Safeguarding these invaluable assets is paramount, and the chosen CDMO must demonstrate a robust and unwavering commitment to IP protection.
Safeguarding Innovations: Robust IP Strategies and Contractual Agreements
The decision to partner with a CDMO involves sharing sensitive intellectual property, from drug formulations and manufacturing processes to analytical methods.75 Therefore, prioritizing intellectual property protection is crucial, and companies must ensure their CDMO partner has a robust IP strategy.65
Key elements to scrutinize in contractual agreements include:
- Clear IP Ownership: The contract must clearly define the ownership of any intellectual property generated during the partnership, whether it’s related to process improvements, new formulations, or analytical methods.76 Ambiguity in this area can lead to costly disputes down the line.
- Confidentiality Agreements: Robust confidentiality agreements are crucial to protect proprietary information shared with the CDMO.78
- Data Integrity and Security: Measures to protect sensitive data and intellectual property within the CDMO’s systems are essential, especially with increasing digitization.59
The anxiety of losing control over intellectual assets and the potential misuse of IP by a third party is a legitimate concern when outsourcing.77 Therefore, a CDMO should have clear policies and procedures for protecting client IP, and due diligence should assess their cybersecurity measures and data management practices.73
Intellectual property protection is not merely a legal formality but a non-negotiable strategic imperative in CDMO partnerships. The explicit mention of IP sharing and the creation of “significant intellectual property” by the CDMO during scale-up 75 highlights a critical risk. This means that robust contractual agreements, especially regarding clear IP ownership and confidentiality, are paramount. The financial implications of IP leakage or disputes can be catastrophic, potentially undermining years of research and development investment. Therefore, clients must conduct thorough due diligence on the CDMO’s internal IP protection policies, cybersecurity measures, and their track record in handling sensitive client data. A CDMO that demonstrates a proactive and transparent approach to IP safeguarding signals its trustworthiness and commitment to a secure, long-term partnership.
Leveraging Patent Intelligence: The Role of DrugPatentWatch in Strategic Planning
In the complex world of pharmaceutical development, patent intelligence plays a vital role in strategic planning, both for pharmaceutical companies and CDMOs. Tools like DrugPatentWatch provide deep knowledge on pharmaceutical drugs, including patents, suppliers, generics, and formulation information.28
For CDMO selection and strategic planning, patent data can offer significant competitive intelligence:
- Pipeline Monitoring: By tracking patent applications from pharmaceutical and biotech companies, CDMOs can identify potential clients whose products are likely to require manufacturing support in the near future, allowing for proactive outreach.80
- Technology Alignment: Patents reveal specific technical challenges and requirements. CDMOs can analyze these to determine where their capabilities align with client needs, enabling highly targeted business development efforts. For instance, a CDMO specializing in highly potent compounds can identify patents describing such compounds to focus their outreach.80
- Development Stage Analysis: The progression of patent filings (from composition of matter to formulation to manufacturing process patents) often signals a product’s development stage, informing the type of CDMO services required.80
DrugPatentWatch, as a platform providing business intelligence on biologic and small molecule drugs, drugs in development, litigation, and patent expirations, can be an invaluable tool for both clients performing due diligence on CDMOs and CDMOs themselves in strategic market positioning.28 It allows for “sector landscaping and due diligence” and helps identify “market entry opportunities”.79 This means that leveraging such patent intelligence allows for more informed decision-making, ensuring that CDMO selection is not just based on current needs but also on future market trends and competitive landscapes.
Real-World Implications and Case Studies
The theoretical framework for CDMO selection gains significant weight when viewed through the lens of real-world applications, highlighting both the triumphs and tribulations of these critical partnerships.
Successful Partnerships: Characteristics and Examples
Successful CDMO partnerships are characterized by a confluence of the key factors discussed: deep expertise, robust quality systems, transparent communication, and a shared commitment to patient impact.69 These collaborations move beyond mere transactions, evolving into synergistic relationships where the CDMO acts as an extension of the client’s team.
For instance, during the COVID-19 pandemic, leading pharmaceutical companies like Moderna and Pfizer collaborated with CDMOs such as Catalent and Lonza to meet the surge in vaccine demand.81 This was not just about capacity; it was about leveraging the CDMOs’ advanced manufacturing capabilities, regulatory expertise, and ability to scale rapidly under immense pressure.9 The success of these partnerships underscored the CDMOs’ critical role in accelerating the production and distribution of life-saving therapies.
Another example can be seen in the mRNA therapeutics CDMO market, which grew from USD 4.62 billion in 2024 to USD 5.15 billion in 2025, with forecasts suggesting a remarkable leap to USD 13.63 billion by 2034.57 This growth has been fueled by biotech and pharma companies increasingly outsourcing the entire lifecycle – from R&D to commercial packaging – to specialized CDMOs offering advanced facilities, scalability, and compliance expertise.57 The success stories here are often implied through the desired characteristics of a CDMO: open communication and accessibility to staff, a collaborative and transparent working relationship, and an appropriate Quality Management System with a proven track record.31 When these elements are in place, CDMOs can significantly streamline drug product manufacturing, as demonstrated by platforms designed to maximize speed and success, taking the burden off the client.82
Common Pitfalls and Lessons Learned from Failed Collaborations
Despite the immense potential, CDMO partnerships are not without their risks. Failures often stem from a range of interconnected issues, where even minor missteps can snowball into significant problems, impacting timelines, budgets, and reputations.25
Common pitfalls include:
- Misaligned Expectations: When contractual agreements lack specificity, both parties may interpret project scope, deliverables, and timelines differently, leading to frustration and strained relationships.25
- Poor Communication: Infrequent or one-way communication can cause small issues to escalate into unmanageable crises, leaving companies blindsided by setbacks.25 A CDMO that is unwilling to have frank conversations about potential risks or delays is a significant red flag.70
- Hidden Costs: Unclear pricing structures or unexpected fees for services like regulatory submissions or quality testing can lead to significant budget overruns.25
- Overpromising Capabilities: Some CDMOs may overpromise on their capabilities to win contracts, which only becomes apparent when work starts, leading to delayed timelines, subpar quality, and expensive deviations.25
- Inadequate Project Management: Poor planning, insufficient resource allocation, and weak oversight disrupt timelines and inflate budgets, especially if plans fail to adapt as projects evolve.25
- Regulatory Non-Compliance: If a CDMO fails to meet stringent regulatory requirements, the consequences for the sponsor company can be severe, ranging from product recalls and costly fines to damaged reputations and lost market opportunities.25
- Lack of Financial Stability: Instances where CDMOs closed partway through a project due to financial issues have been reported, abruptly halting production and causing significant delays.61
- Intellectual Property Concerns: Outsourcing can fragment end-to-end knowledge of a drug lifecycle and raise concerns about IP ownership and potential misuse, especially if agreements are not meticulously defined.77
The lesson from these failures is clear: due diligence is not a formality but a continuous, in-depth process. Both sponsors and CDMOs must ensure they complete their due diligence to avoid such scenarios and maintain reputational integrity throughout the project.25 Learning from these pitfalls and implementing best practices, such as establishing clear communication plans and robust governance structures, is essential for transforming potential obstacles into opportunities for growth and innovation.12
The Future of CDMO Partnerships
The CDMO industry is not static; it is a dynamic sector constantly evolving to meet the complex demands of pharmaceutical innovation. Understanding the trajectory of these changes is crucial for strategic planning.
Market Trends and Future Outlook
The global pharmaceutical CDMO market is experiencing sustained growth, projected to reach USD 368.7 billion by 2034, with a CAGR of approximately 6.9%.53 This expansion is fueled by an increasing number of companies embracing outsourcing to achieve cost efficiencies, scalability, and access to specialized expertise.6 Outsourcing is becoming a strategic imperative for firms of all sizes; large pharma uses it to de-risk supply chains and complement internal capabilities, while smaller firms outsource due to limited in-house infrastructure.53
Key trends shaping the future include:
- Continued Demand for Biologics and Advanced Therapies: The rising prevalence of chronic diseases and the increasing demand for biologic therapies, such as monoclonal antibodies, cell and gene therapies, and mRNA therapeutics, are driving significant growth in the biologics CDMO market.17
- Technological Advancements: CDMOs will continue to invest heavily in advanced manufacturing technologies like continuous manufacturing, automation, AI, and digital transformation to boost efficiency, reduce costs, and improve product quality.53 AI, in particular, is expected to revolutionize production processes, predictive maintenance, and quality control.53
- Industry Consolidation: The CDMO landscape is still fragmented, with the top five players controlling only 15% of the market.53 Mergers and acquisitions (M&As) are expected to continue, as larger CDMOs acquire smaller firms to gain access to specialist drug delivery technologies, expand capabilities, and offer integrated, end-to-end service portfolios.9
- Regional Dynamics: While North America remains a key hub, the Asia-Pacific region is experiencing rapid growth in the CDMO market.74 However, geopolitical tensions and policies like the BioSecure Act are prompting a re-evaluation of global supply chains and a trend towards reshoring manufacturing.8
The overall outlook for the CDMO sector remains positive, as biopharma companies increasingly embrace outsourcing trends, technological advancements, and strategic consolidation efforts.53
Evolving Regulatory Landscape and Its Impact
The regulatory environment governing pharmaceutical manufacturing is becoming increasingly complex, posing significant challenges for CDMOs.8 Stricter regulations demand continuous adaptation and proactive compliance. CDMOs must invest in regulatory intelligence tools to stay ahead of evolving legislation and enhance data security measures to meet stringent global standards.59
Regulatory bodies like the FDA are also evolving their approach to inspections, moving beyond mere compliance checklists to assess a company’s “quality culture” and “maturity of the process development program”.39 This means CDMOs need to demonstrate not just adherence to cGMPs but also a commitment to continuous improvement and a proactive approach to risk management.37 The ability to navigate these complexities, ensuring fulfillment of regulatory guidelines established by authorities like the FDA and EMA, is crucial for reducing the risk of delays due to regulatory approval concerns and bringing products to market faster.4
Strategic Consolidation and Specialization
The CDMO industry is witnessing a wave of strategic consolidation, driven by the need for scale, specialized capabilities, and integrated service offerings. Larger CDMOs are acquiring smaller, specialized firms to broaden their portfolios and create “one-stop-shop” solutions that support the entire drug lifecycle, from early-stage design to commercialization.6 This trend is particularly evident in high-growth areas like cell and gene therapies and antibody-drug conjugates, which require significant investment in new manufacturing capabilities.8
This consolidation aims to streamline production workflows, eliminate technology transfer risks, and expedite time-to-market by integrating services under one roof.2 However, even with consolidation, specialization remains key. The market is bifurcating, with “hot spots” of innovation requiring deeper technical capabilities.8 CDMOs are increasingly focusing on niche areas, such as high-potency API manufacturing or specific dosage forms, to offer differentiated value.8 The “Goldilocks” positioning – large enough to scale, small enough to move fast – is becoming a competitive advantage, allowing CDMOs to support customers across the full product lifecycle while remaining agile.8 This strategic consolidation, coupled with ongoing specialization, will continue to redefine the CDMO landscape, offering pharmaceutical companies more integrated yet highly specialized partnership options.
Conclusion: Transforming Data into Market Domination
The journey of bringing a pharmaceutical product to market is a complex, capital-intensive, and inherently risky endeavor. In this high-stakes environment, the strategic selection of a Contract Development and Manufacturing Organization (CDMO) transcends a mere operational decision; it becomes a pivotal determinant of a company’s competitive advantage and its path to market domination.
The analysis reveals that CDMOs have evolved from transactional service providers to indispensable strategic partners. This transformation is driven by the escalating complexity of novel drug modalities, the prohibitive costs of in-house infrastructure, and the relentless pressure for accelerated time-to-market. A well-chosen CDMO offers not just manufacturing capacity, but a symbiotic relationship built on shared expertise, advanced technology, and a mutual commitment to quality and patient impact.
Key factors for selection, such as a CDMO’s unwavering commitment to quality, its deep and relevant expertise across specific molecule types, and its embrace of cutting-edge technologies like continuous manufacturing, 3D printing, and AI-driven automation, are no longer optional but essential. Furthermore, a CDMO’s scalable capacity, operational flexibility, transparent communication, robust project management, and unwavering financial stability are critical for navigating the unpredictable landscape of drug development. Finally, the meticulous protection of intellectual property and the resilience of global supply chains are non-negotiable pillars that safeguard a company’s most valuable assets and ensure uninterrupted market access.
For business professionals aiming to transform data into market domination, the message is clear: rigorous due diligence, a granular understanding of internal needs, and a proactive approach to partnership cultivation are paramount. By leveraging the comprehensive capabilities of the right CDMO, companies can optimize resource allocation, mitigate risks, accelerate innovation, and ultimately, bring life-changing therapies to patients faster and more efficiently. This strategic alignment is the true blueprint for success in the modern pharmaceutical industry.
Key Takeaways
- CDMOs are Strategic Partners, Not Just Vendors: The relationship has evolved from transactional outsourcing to deeply integrated, collaborative partnerships essential for navigating complex drug development.
- Relevance of Expertise is Paramount: A CDMO’s experience must specifically align with the client’s molecule type, therapeutic area, and development stage, not just general industry longevity.
- Quality Culture Trumps Mere Compliance: Beyond regulatory adherence, a CDMO’s proactive quality management system, transparency, and empowered workforce indicate a true commitment to product integrity and patient safety.
- Advanced Technology is a Differentiator: CDMOs investing in continuous manufacturing, 3D printing, AI, and automation offer significant advantages in speed, efficiency, customization, and risk mitigation.
- Capacity and Flexibility are Critical for Agility: A CDMO must demonstrate not only current capacity but also a robust plan for scaling up (or down) and mitigating supply chain disruptions through diversification and strategic sourcing.
- Transparency Builds Trust: Clear communication, dedicated project managers, and open dialogue are foundational for effective collaboration and problem-solving throughout the project lifecycle.
- Financial Stability is a Long-Term Assurance: A CDMO’s financial health directly impacts its ability to sustain operations, invest in innovation, and reliably deliver services, making financial due diligence crucial.
- Intellectual Property Protection is Non-Negotiable: Robust contractual agreements and a CDMO’s proven IP safeguarding strategies are essential to protect valuable innovations.
5 Unique FAQs
- How does the increasing complexity of new drug modalities, such as cell and gene therapies, specifically influence the selection criteria for a CDMO?
The increasing complexity of new drug modalities necessitates CDMOs with highly specialized expertise, equipment, and a proven track record in handling these unique molecules. General experience in traditional small molecules is often insufficient; instead, companies must seek CDMOs with specific, relevant experience in areas like viral vector manufacturing, stringent chain-of-custody requirements for living materials, and advanced analytical development tailored to these complex therapies. This focus on specialized capability over broad experience significantly narrows the field of suitable partners. - Beyond cost savings, what quantifiable long-term ROI can a pharmaceutical company expect from a strategically chosen CDMO partnership, particularly concerning market entry and competitive advantage?
Beyond direct cost savings from avoiding CAPEX, a strategically chosen CDMO can deliver significant long-term ROI by accelerating time-to-market, which directly translates to earlier revenue generation and increased market share. For example, a CDMO’s ability to streamline development and regulatory processes can shave months or even years off a drug’s journey to approval, allowing a company to capture first-mover advantage. This accelerated market entry, coupled with reduced risks of costly delays or recalls due to the CDMO’s quality systems, enhances a company’s competitive position and maximizes the lifetime value of its drug pipeline. - In what specific ways can a CDMO’s adoption of AI and automation fundamentally alter the risk profile of a drug development project, moving beyond mere efficiency gains?
A CDMO’s adoption of AI and automation fundamentally alters a drug development project’s risk profile by shifting from reactive problem-solving to proactive, predictive optimization. AI can predict potential issues like formulation instability or equipment failures before they occur, enabling preventive measures. Automated systems and AI agents can monitor production in real-time, detect deviations instantly, and even self-correct, significantly reducing the risk of batch failures or quality excursions. This intelligent oversight minimizes human error, ensures consistent product quality, and provides a more resilient and predictable development pathway, thereby de-risking the entire project. - How should a pharmaceutical company interpret a CDMO’s history of FDA Form 483 observations versus Warning Letters during due diligence, and what do these reveal about the CDMO’s underlying quality culture?
An FDA Form 483 indicates observations that may constitute violations, serving as a guide for voluntary corrective action. A Warning Letter, however, signifies a confirmed violation and is a much more serious public document, often issued when 483 issues were not adequately addressed. During due diligence, a history of numerous or recurring 483s, especially if they escalate to Warning Letters, reveals deeper systemic issues within the CDMO’s quality management system, operational maturity, and quality culture. It suggests a reactive approach to compliance rather than a proactive commitment to quality by design, indicating potential risks for future projects. - With increasing geopolitical volatility and supply chain disruptions, what innovative strategies are leading CDMOs implementing to ensure supply chain resilience for their clients, beyond traditional diversification?
Beyond traditional diversification, leading CDMOs are implementing innovative strategies such as dual-sourcing critical raw materials from different geographical regions to build redundancy. They are also investing in domestic or regional manufacturing capabilities (reshoring) to reduce reliance on distant supply chains and mitigate risks from tariffs or geopolitical tensions. Furthermore, some CDMOs are proactively maintaining strategic stock of essential reagents and materials in controlled storage facilities, and leveraging advanced data analytics and AI for real-time supply chain monitoring and predictive risk assessment, ensuring greater agility and continuity of supply for their clients.
Works cited
- What is a CDMO & How Do They Help Pharma Companies? – Medical Packaging Inc, accessed July 16, 2025, https://medpak.com/cdmo-pharma/
- CROs vs CMOs, and CDMOs: What’s the difference between the three? – Patheon, accessed July 16, 2025, https://www.patheon.com/us/en/insights-resources/blog/cdmo-vs-cmo-vs-cro.html
- CROs vs. CMOs vs. CDMOs: Understanding the Differences in Outsourcing Partners, accessed July 16, 2025, https://cellcarta.com/science-hub/cro-vs-cmo-vs-cdmo/
- The Role of CDMOs in Accelerating Time-to-Market for Pharma Innovations, accessed July 16, 2025, https://globelapharma.com/the-role-of-cdmos-in-accelerating-time-to-market-for-pharma-innovations/
- What is a CDMO? Services, Benefits and Impact of CDMOs, accessed July 16, 2025, https://renejix.com/what-is-cdmo/
- CDMO Market Size, Share & Trends | Growth Analysis [2032] – Fortune Business Insights, accessed July 16, 2025, https://www.fortunebusinessinsights.com/contract-development-and-manufacturing-organization-cdmo-outsourcing-market-102502
- CDMO Services: Top Solutions to Transform Your Pharma Journey, accessed July 16, 2025, https://adragos-pharma.com/cdmo-services-top-solutions-to-transform-your-pharma-journey/
- CDMOs: the changing shape of outsourcing – Investors in Healthcare, accessed July 16, 2025, https://www.investorsinhealthcare.com/articles/category/analysis/cdmos-the-changing-shape-of-outsourcing/
- CDMOs are becoming emerging technology leaders | EY – US, accessed July 16, 2025, https://www.ey.com/en_us/insights/strategy/how-cdmo-companies-are-leading-innovation-for-pharmaceutical-partners
- The 5 Drug Development Phases – Patheon pharma services, accessed July 16, 2025, https://www.patheon.com/us/en/insights-resources/blog/drug-development-phases.html
- How Pharmaceutical Companies Choose A CDMO Part 1 – Outsourced Pharma, accessed July 16, 2025, https://www.outsourcedpharma.com/doc/how-pharmaceutical-companies-choose-a-cdmo-part-1-0001
- 7 Focus Areas To Establish A Win-Win Relationship With Your CDMO – Outsourced Pharma, accessed July 16, 2025, https://www.outsourcedpharma.com/doc/focus-areas-to-establish-a-win-win-relationship-with-your-cdmo-0001
- CDMO Case Study – Athena SWC | Lead Generation for Manufacturing Companies, accessed July 16, 2025, https://www.athenaswc.com/case-studies/cdmo-case-study/
- Quality as a Catalyst: How Risk Culture and Transparency Drive CDMO Success, accessed July 16, 2025, https://pharmasource.global/content/quality-as-catalyst-how-risk-culture-and-transparency-drive-cdmo-success/
- Aligning CDMO Services with Business Goals: Strategies for Success – DrugPatentWatch, accessed July 16, 2025, https://www.drugpatentwatch.com/blog/aligning-cdmo-services-with-business-goals-strategies-for-success/
- CDMO Support: Success in Early Phase Drug Product Development & Manufacturing, accessed July 16, 2025, https://pci.com/resources/cdmo-early-phase-drug-product-development-and-manufacturing/
- U.S. Pharmaceutical CDMO Market Size to Hit USD 83.86 Billion by 2034 – BioSpace, accessed July 16, 2025, https://www.biospace.com/press-releases/u-s-pharmaceutical-cdmo-market-size-to-hit-usd-83-86-billion-by-2034
- The CDMO Surge: Why Pharma Is Outsourcing More Than Ever – News-Medical.net, accessed July 16, 2025, https://www.news-medical.net/life-sciences/The-CDMO-Surge-Why-Pharma-Is-Outsourcing-More-Than-Ever.aspx
- The Strategic Value of a CDMO in the Biopharmaceutical Industry – 53Biologics, accessed July 16, 2025, https://53biologics.com/blog/the-strategic-value-of-a-cdmo-in-the-pharmaceutical-industry/
- What Is a Contract Development & Manufacturing Organization? – Moravek, accessed July 16, 2025, https://www.moravek.com/what-is-a-contract-development-and-manufacturing-organization/
- CDMO or no CDMO: That is the question – Cytiva, accessed July 16, 2025, https://www.cytivalifesciences.com/en/us/solutions/bioprocessing/knowledge-center/pulse-check
- Biologics CDMO Global and Regional Market Analysis Report 2025-2035: Outsourcing Trend Accelerates as Biologics CDMOs Offer Speed, Scale, and Regulatory Expertise – ResearchAndMarkets.com – Business Wire, accessed July 16, 2025, https://www.businesswire.com/news/home/20250526408574/en/Biologics-CDMO-Global-and-Regional-Market-Analysis-Report-2025-2035-Outsourcing-Trend-Accelerates-as-Biologics-CDMOs-Offer-Speed-Scale-and-Regulatory-Expertise—ResearchAndMarkets.com
- CDMOs and How They Support Business Development – Akadeum Life Sciences, accessed July 16, 2025, https://www.akadeum.com/blog/what-is-a-cdmo/
- Choosing A CDMO: Key Considerations – PCI Pharma Services, accessed July 16, 2025, https://pci.com/resources/choosing-a-cdmo-key-considerations/
- What Happens When Your CDMO Partnership Goes Wrong? – Patrick Wareing, accessed July 16, 2025, https://patrickwareing.com/news/what-happens-when-your-cdmo-partnership-goes-wrong/
- Top 10 Factors to Consider When Choosing a CDMO, accessed July 16, 2025, https://renejix.com/criteria-for-cdmo-selection/
- Key considerations when choosing a CDMO for your project – News-Medical.net, accessed July 16, 2025, https://www.news-medical.net/whitepaper/20250312/Key-considerations-when-choosing-a-CDMO-for-your-project.aspx
- CDMO Selection: The Ultimate Checklist – DrugPatentWatch …, accessed July 16, 2025, https://www.drugpatentwatch.com/blog/cdmo-selection-the-ultimate-checklist/
- Preparing For Your CDMO Selection Process | Contract Pharma, accessed July 16, 2025, https://www.contractpharma.com/preparing-for-your-cdmo-selection-process-762227/
- Preparing for Your CDMO Selection Process | Aji Bio-Pharma, accessed July 16, 2025, https://ajibio-pharma.com/wp-content/uploads/2018/09/Aji-Bio-Pharma_Preparing-for-Your-CDMO-Selection-Process.pdf
- Outsourcing to accelerate your next milestone | Cytiva, accessed July 16, 2025, https://www.cytivalifesciences.com/en/us/solutions/emerging-biotech/knowledge-center/cdmo-checklist
- Questions You And Your New CDMO Might Be Asking – Outsourced Pharma, accessed July 16, 2025, https://www.outsourcedpharma.com/doc/questions-you-and-your-new-cdmo-might-be-asking-0001
- Five Qualities to Look for in a CDMO – VGXI, accessed July 16, 2025, https://vgxii.com/five-qualities-to-look-for-in-a-cdmo/
- How to Choose the Right CDMO Partner: Key Factors to Consider, accessed July 16, 2025, https://www.bachem.com/articles/blog/how-to-choose-the-right-cdmo-partner-key-factors-to-consider/
- The Ultimate Audit Checklist for Pharmaceutical Inspections – – Pharmuni, accessed July 16, 2025, https://pharmuni.com/2025/02/10/the-ultimate-audit-checklist-for-pharmaceutical-inspections/
- How to Evaluate a CDMO’s Commitment to Quality – Pharmaceutical Technology, accessed July 16, 2025, https://www.pharmtech.com/view/how-to-evaluate-a-cdmo-s-commitment-to-quality
- How to Achieve CDMO Operational Excellence – DrugPatentWatch, accessed July 16, 2025, https://www.drugpatentwatch.com/blog/how-to-achieve-cdmo-operational-excellence/
- Regulatory Inspections for Pharma Companies, accessed July 16, 2025, https://www.qualityze.com/blogs/regulatory-inspections-for-pharma-companies
- Quality Culture Wins Over Compliance | American Pharmaceutical Review, accessed July 16, 2025, https://www.americanpharmaceuticalreview.com/Featured-Articles/187437-Quality-Culture-Wins-Over-Compliance/
- The Definitive Guide to Responding to FDA 483 Observations and Warning Letters, accessed July 16, 2025, https://www.greenlight.guru/blog/fda-483-warning-letters
- DA Compliance: A Framework for Choosing a Quality CMO, accessed July 16, 2025, https://oakwoodlabs.com/fda-compliance-a-framework-for-choosing-a-quality-cmo/
- FDA 483 and Warning Letters: What Pharma Companies Need to Know – Drugzone, accessed July 16, 2025, https://www.drugzone.com/blog/fda-483-and-warning-letters-what-pharma-companies-need-to-know
- Responding to an FDA 483 or an FDA Warning Letter – Kellerman Consulting, accessed July 16, 2025, https://kellermanconsulting.com/responding-to-an-fda-483-or-an-fda-warning-letter/
- Critical Differences Between a Form FDA 483 and Warning Letters – Parexel, accessed July 16, 2025, https://www.parexel.com/insights/blogs/critical-differences-between-a-form-fda-483-and-warning-letters-4
- www.drugpatentwatch.com, accessed July 16, 2025, https://www.drugpatentwatch.com/blog/cdmo-vendor-management-best-practices/#:~:text=Quality%20and%20Regulatory%20Compliance%3A%20Assess,and%20compliance%20with%20regulatory%20requirements.
- CDMO Pharma & Contract Manufacturers – Regulatory Compliance Associates, accessed July 16, 2025, https://www.rcainc.com/published-articles/cdmo-pharma/
- CDER Quality Management Maturity | FDA, accessed July 16, 2025, https://www.fda.gov/drugs/pharmaceutical-quality-resources/cder-quality-management-maturity
- Continuous manufacturing (flow chemistry) – the future of pharmaceutical manufacturing, accessed July 16, 2025, https://veranova.com/expert-insights/continuous-manufacturing-the-future-of-pharmaceutical-manufacturing/
- Continuous Manufacturing: To Be Continued… | American Pharmaceutical Review, accessed July 16, 2025, https://www.americanpharmaceuticalreview.com/Featured-Articles/335691-Continuous-Manufacturing-To-Be-Continued/
- SPECIAL FEATURE- Outsourcing Formulation Development & Manufacturing: CDMOs Are Making Their Supply Chains More Resilient & Secure, accessed July 16, 2025, https://drug-dev.com/special-feature-outsourcing-formulation-development-manufacturing-cdmos-are-making-their-supply-chains-more-resilient-secure/
- 3D Drug Printing: Revolutionizing Drug Fabrication – YouTube, accessed July 16, 2025, https://www.youtube.com/watch?v=hEmrkHOf23Y
- Exploring New Possibilities with 3D Printing of Pharmaceuticals – Pharma’s Almanac, accessed July 16, 2025, https://www.pharmasalmanac.com/articles/exploring-new-possibilities-with-3d-printing-of-pharmaceuticals
- Five Trends That Will Redefine the CDMO Industry in 2025 – The Pharma Navigator, accessed July 16, 2025, https://www.thepharmanavigator.com/news/five-trends-that-will-redefine-the-cdmo-industry-in-2025
- How CDMOs are Creating Strategic Advantages with Emerging Technologies, accessed July 16, 2025, https://www.tabletscapsules.com/3641-Technical-Articles/618272-How-CDMOs-are-Creating-Strategic-Advantages-with-Emerging-Technologies/
- Future-Proofing Life Sciences Manufacturing 2025 CDMO Trends – Cell and Gene, accessed July 16, 2025, https://www.cellandgene.com/doc/future-proofing-life-sciences-manufacturing-cdmo-trends-0001
- Unleashing the Power of AI // PCI Services / Let’s Talk Future, accessed July 16, 2025, https://pci.com/resources/power-of-ai/
- mRNA Therapeutics CDMO Market Case Study : Trends, Technology, and Growth Outlook (2025–2034) – Healthcare Web Wire, accessed July 16, 2025, https://www.healthcarewebwire.com/mrna-therapeutics-cdmo-market-case-study/
- The Intelligent CDMO A Vision with Agentic AI – Outsourced Pharma, accessed July 16, 2025, https://www.outsourcedpharma.com/doc/the-intelligent-cdmo-a-vision-with-agentic-ai-0001
- The Challenges CDMOs Face in 2025 – And How to Tackle Them – Mint CRM, accessed July 16, 2025, https://www.mintcrm.co.uk/articles/detail/cdmo-challenges-2025/
- A Shifting CDMO Landscape: The Rise of High-Science, High-Touch – Applied Clinical Trials, accessed July 16, 2025, https://www.appliedclinicaltrialsonline.com/view/a-shifting-cdmo-landscape-the-rise-of-high-science-high-touch
- 5 Red Flags to Look Out For When Hiring a MedTech CDMO – Rev.1 Engineering, accessed July 16, 2025, https://rev1engineering.com/blog/5-red-flags-to-look-out-for-when-hiring-a-medtech-cdmo/
- CDMOs Are Making Their Supply Chains More Resilient & Secure, accessed July 16, 2025, https://resilience.com/blog/special-feature-outsourcing-formulation-development-manufacturing
- Redefining procurement in CDMO supply chains: A strategic force in pharmaceutical manufacturing – Sterling Pharma Solutions, accessed July 16, 2025, https://www.sterlingpharmasolutions.com/knowledge-hub/api-manufacturing/redefining-procurement-in-cdmo-supply-chains/
- Outsourcing Formulation Development & Manufacturing: CDMOs Are Making Their Supply Chains More Resilient and Secure – Aragen Life Sciences, accessed July 16, 2025, https://www.aragen.com/news/outsourcing-formulation-development-manufacturing-cdmos-are-making-their-supply-chains-more-resilient-and-secure/
- Analysis Highlights Five Key Factors for Successful Pharma-CDMO Partnerships, accessed July 16, 2025, https://www.geneonline.com/analysis-highlights-five-key-factors-for-successful-pharma-cdmo-partnerships/
- Best Practices for CDMO Evaluation, Selection, and Management – Project Farma, accessed July 16, 2025, https://www.projectfarma.com/wp-content/uploads/2020/07/CDMO-Management-06-24-20-Final_compressed.pdf
- CDMO project management: executing biopharmaceutical CDMO projects with customer focus | Richter BioLogics, accessed July 16, 2025, https://www.richterbiologics.eu/cdmo-project-management-executing-biopharmaceutical-cdmo-projects-with-customer-focus/
- 6 Common Pitfalls in CDMO Partnerships—and How to Avoid Them – List Labs, accessed July 16, 2025, https://listlabs.com/6-common-pitfalls-in-cdmo-partnerships-and-how-to-avoid-them/
- CDMO Project Success Stories That Put Patients First – YouTube, accessed July 16, 2025, https://www.youtube.com/watch?v=fTA6ZqYbEmI
- 4 Steps to Evaluating a CDMO Proposal | Mabion, accessed July 16, 2025, https://www.mabion.eu/science-hub/articles/four-steps-to-identifying-the-cdmo-proposal-worth-signing/
- Uncovering Hidden Risks and Value – Quality Executive Partners, accessed July 16, 2025, https://www.qualityexecutivepartners.com/thought-leadership/uncovering-hidden-risks-and-value
- The Ultimate Guide to CDMO Pricing – DrugPatentWatch …, accessed July 16, 2025, https://www.drugpatentwatch.com/blog/the-ultimate-guide-to-cdmo-pricing/
- Due Diligence Questionnaire — the Ultimate Guide – Allegrow, accessed July 16, 2025, https://allegrow.com/due-diligence-questionnaire/
- Healthcare Contract Development and Manufacturing Organization Market – 2032, accessed July 16, 2025, https://www.transparencymarketresearch.com/healthcare-contract-development-and-manufacturing-organization-market.html
- Innovator Checklist for Finding Contract Manufacturing Organization (CMO) – NIH’s Seed, accessed July 16, 2025, https://seed.nih.gov/sites/default/files/2023-12/Innovator-Checklist-for-Finding-Contract-Manufacturing-Organization.pdf
- CDMO Contracts: Clear Project Goals and IP Protection are Essential for Pharmaceutical Companies to Mitigate Project Failure Risks. – GeneOnline, accessed July 16, 2025, https://www.geneonline.com/cdmo-contracts-clear-project-goals-and-ip-protection-are-essential-for-pharmaceutical-companies-to-mitigate-project-failure-risks/
- Challenges And Opportunities Of Outsourcing Biopharma Development – Bioprocess Online, accessed July 16, 2025, https://www.bioprocessonline.com/doc/challenges-and-opportunities-of-outsourcing-biopharma-development-0001
- Best CDMO Practices for Startups: Navigating the Complex World of Contract Development and Manufacturing – DrugPatentWatch – Transform Data into Market Domination, accessed July 16, 2025, https://www.drugpatentwatch.com/blog/best-cdmo-practices-for-startups-navigating-the-complex-world-of-contract-development-and-manufacturing/
- DrugPatentWatch | Software Reviews & Alternatives – Crozdesk, accessed July 16, 2025, https://crozdesk.com/software/drugpatentwatch
- How CDMOs Can Use Patent Data to Win More Pharmaceutical Clients – DrugPatentWatch, accessed July 16, 2025, https://www.drugpatentwatch.com/blog/how-cdmos-can-use-patent-data-to-win-more-pharmaceutical-clients/
- Optimizing Outsourcing: A Case Study on Evaluating CDMO Capabilities for API and FD Development – PR Newswire, accessed July 16, 2025, https://www.prnewswire.com/news-releases/optimizing-outsourcing-a-case-study-on-evaluating-cdmo-capabilities-for-api-and-fd-development-302341149.html
- CDMO CASE STUDY – AJILITY: Streamlining Drug Product Manufacturing, accessed July 16, 2025, https://drug-dev.com/cdmo-case-study-ajility-streamlining-drug-product-manufacturing/