MESALAMINE Drug Patent Profile
✉ Email this page to a colleague
When do Mesalamine patents expire, and when can generic versions of Mesalamine launch?
Mesalamine is a drug marketed by Teva Pharms Usa, Alembic, Alkem Labs Ltd, Amta, Annora Pharma, Aurobindo Pharma Ltd, Mylan, Novast Labs, Sun Pharm, Zydus Pharms, Encube, G And W Labs Inc, Novitium Pharma, Padagis Israel, Actavis Mid Atlantic, Amneal, Amring Pharms, Quagen, Rising, Sandoz, Actavis Labs Fl, Sinotherapeutics Inc, and Teva Pharms Inc. and is included in thirty-three NDAs.
The generic ingredient in MESALAMINE is mesalamine. There are twenty-eight drug master file entries for this compound. Thirty-eight suppliers are listed for this compound. Additional details are available on the mesalamine profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Mesalamine
A generic version of MESALAMINE was approved as mesalamine by PADAGIS ISRAEL on September 17th, 2004.
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for MESALAMINE?
- What are the global sales for MESALAMINE?
- What is Average Wholesale Price for MESALAMINE?
Summary for MESALAMINE
| US Patents: | 0 |
| Applicants: | 23 |
| NDAs: | 33 |
| Finished Product Suppliers / Packagers: | 30 |
| Raw Ingredient (Bulk) Api Vendors: | 130 |
| Clinical Trials: | 104 |
| Patent Applications: | 4,400 |
| Drug Prices: | Drug price information for MESALAMINE |
| Drug Sales Revenues: | Drug sales revenues for MESALAMINE |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for MESALAMINE |
| What excipients (inactive ingredients) are in MESALAMINE? | MESALAMINE excipients list |
| DailyMed Link: | MESALAMINE at DailyMed |
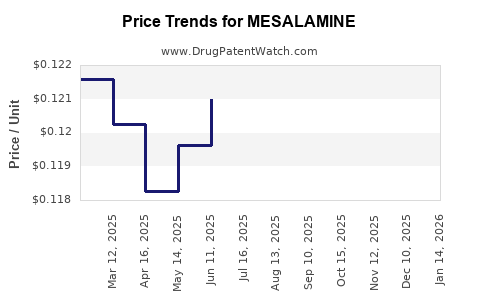
See drug prices for MESALAMINE
Recent Clinical Trials for MESALAMINE
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Grupo Espanol de Trabajo en Enfermedad de Crohn y Colitis Ulcerosa | PHASE4 |
| Prof. Dr. Ahmed Ibrahim Mohammed El Mallah, faculty of pharmacy, Alexandria University. | PHASE2 |
| Assoc. Prof. Dr. Noha Alaa Eldin Hassan Hamdy, Faculty of pharmacy, Alexandria University. | PHASE2 |
Pharmacology for MESALAMINE
| Drug Class | Aminosalicylate |
Medical Subject Heading (MeSH) Categories for MESALAMINE
Paragraph IV (Patent) Challenges for MESALAMINE
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| DELZICOL | Delayed-release Capsules | mesalamine | 400 mg | 204412 | 1 | 2014-06-17 |
| CANASA | Suppository | mesalamine | 1000 mg | 021252 | 1 | 2013-05-24 |
| APRISO | Extended-release Capsules | mesalamine | 0.375 g | 022301 | 1 | 2012-04-03 |
| ASACOL HD | Delayed-release Tablets | mesalamine | 800 mg | 021830 | 1 | 2011-07-13 |
| LIALDA | Delayed-release Tablets | mesalamine | 1.2 g | 022000 | 1 | 2009-12-16 |
| ASACOL | Delayed-release Tablets | mesalamine | 400 mg | 019651 | 1 | 2007-06-22 |
US Patents and Regulatory Information for MESALAMINE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zydus Pharms | MESALAMINE | mesalamine | TABLET, DELAYED RELEASE;ORAL | 091640-001 | Jun 5, 2017 | AB | RX | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Mylan | MESALAMINE | mesalamine | TABLET, DELAYED RELEASE;ORAL | 203574-001 | Nov 20, 2018 | DISCN | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Zydus Pharms | MESALAMINE | mesalamine | TABLET, DELAYED RELEASE;ORAL | 203286-001 | Jul 21, 2017 | AB | RX | No | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Sinotherapeutics Inc | MESALAMINE | mesalamine | TABLET, DELAYED RELEASE;ORAL | 217337-001 | May 12, 2023 | AB | RX | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Teva Pharms Usa | MESALAMINE | mesalamine | CAPSULE, DELAYED RELEASE;ORAL | 207873-001 | May 9, 2019 | RX | No | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Amring Pharms | MESALAMINE | mesalamine | SUPPOSITORY;RECTAL | 208362-001 | Jun 21, 2019 | AB | RX | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Zydus Pharms | MESALAMINE | mesalamine | SUPPOSITORY;RECTAL | 208953-001 | Feb 12, 2020 | AB | RX | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Market Dynamics and Financial Trajectory for MESALAMINE
More… ↓
