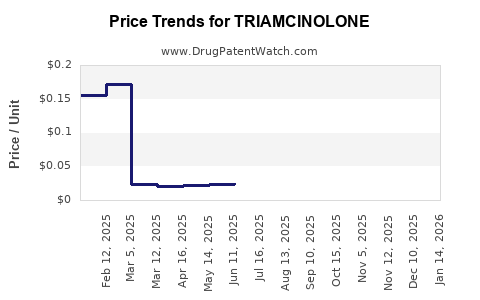
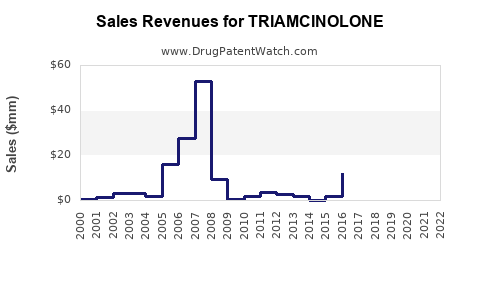
Last updated: July 27, 2025
Introduction
Triamcinolone is a synthetic corticosteroid utilized extensively across dermatology, dentistry, allergy, and respiratory medicine. Its anti-inflammatory and immunosuppressive properties have established it as a versatile therapeutic agent. As the pharmaceutical landscape evolves, understanding the market dynamics and financial outlook surrounding triamcinolone is vital for stakeholders, including manufacturers, investors, and healthcare policymakers.
Therapeutic Applications and Market Penetration
Triamcinolone's primary indications include allergic reactions, skin disorders, arthritis, and nasal polyps. Its formulations—topical, injectable, and nasal spray—contribute to widespread adoption. The topical form constitutes a significant market share due to chronic dermatological conditions' prevalence, such as eczema and psoriasis.
The emergence of biosimilars and generics has expanded accessibility and reduced treatment costs, boosting prescribing rates globally. For instance, the advent of lower-cost triamcinolone-based products in emerging markets has helped penetrate resource-constrained healthcare systems, fostering future growth.
Market Drivers
Growing Prevalence of Target Diseases
Rising incidences of allergy-related disorders, autoimmune skin diseases, and respiratory conditions are driving demand. According to the WHO, asthma affects over 300 million people worldwide, with corticosteroids like triamcinolone as cornerstone treatments [1].
Expanding Dermatological Market
The increasing prevalence of skin conditions, compounded by aging populations, creates an expanding customer base for topical corticosteroids. The rise in cosmetic dermatology procedures further fuels demand for anti-inflammatory agents like triamcinolone.
Product Approvals and Formulation Innovations
Regulatory approvals for novel formulations—such as long-acting injectable triamcinolone acetonide—enhance patient compliance and extend market reach. Ongoing research into combination therapies and sustained-release systems indicate future diversification.
Healthcare Infrastructure Development
Improving healthcare infrastructure in emerging economies boosts access to corticosteroid therapies. The proliferation of outpatient clinics and dermatology centers supports increased prescription rates, positively influencing revenue streams.
Market Challenges
Regulatory and Patent Landscape
Stringent regulations around corticosteroids due to potential side effects, such as skin atrophy and hormonal imbalances, may impose restrictions. Patent expirations have led to the proliferation of generics, exerting downward pressure on prices and margins.
Side Effect Profile and Physician Preference
While effective, corticosteroids' adverse effects can limit long-term use. Increasing physician preference for steroid-sparing agents can hinder sales growth, especially as new biologics and targeted therapies emerge.
Market Saturation and Competition
The presence of multiple corticosteroids with similar indications leads to intense competition. Differentiation hinges on formulation efficacy, delivery systems, and cost, challenging manufacturers to innovate continuously.
Financial Trajectory and Market Forecast
Historical Performance
From 2015 to 2020, the global corticosteroid market—including triamcinolone—experienced a compound annual growth rate (CAGR) of approximately 4.5%. Key contributors included North America, Europe, and the Asia-Pacific region, driven by chronic disease prevalence and expanding healthcare infrastructure [2].
Projected Growth
The market is projected to grow at a CAGR of 5.2% between 2023 and 2030, reaching an estimated valuation of USD 1.8 billion by 2030. The Asia-Pacific region is anticipated to account for the largest regional share due to rising affordability, increased awareness, and pharmaceutical manufacturing expansion [3].
Segment Analysis
- Formulation Type: Topical formulations are expected to continue dominant, but injectable forms are poised for growth owing to their use in severe dermatological and inflammatory conditions.
- Application Area: Dermatology applications will lead growth, leveraging increasing skin disease prevalence.
- End-User: Hospitals and clinics will remain primary distribution channels, especially in emerging economies.
Market Influencers
Factors such as patent expiries of branded products, emerging biosimilars, and cost-sensitive markets will influence pricing strategies and revenue streams. Strategic collaborations and regional market entries will be pivotal for growth.
Competitive Landscape
Major players include Pfizer, Sandoz (Novartis), Teva Pharmaceuticals, and Mylan, offering various formulations of triamcinolone. Patent expirations stimulate new entrants and generic manufacturing, intensifying market competition. Innovation focus, such as sustained-release delivery systems, will be critical differentiators.
Regulatory and Ethical Considerations
Regulatory agencies (FDA, EMA, etc.) emphasize safety, efficacy, and quality. Recalls and warnings related to corticosteroid side effects necessitate stringent post-marketing surveillance. Ethical marketing practices and transparent clinical data are increasingly mandated, impacting commercialization strategies.
Conclusion
Triamcinolone's market dynamics stem from its established therapeutic efficacy, expanding disease prevalence, and formulation innovations. While growth prospects remain favorable, challenges like regulatory constraints, side effect concerns, and fierce generic competition shape the market's financial trajectory.
Stakeholders should heed regional market nuances, prioritize formulation advancements, and adapt to evolving regulatory landscapes to capitalize on upcoming opportunities. Driven by demographic trends and healthcare infrastructure improvements, the triamcinolone market is poised for steady expansion over the next decade.
Key Takeaways
- The global triamcinolone market is projected to grow at a CAGR of approximately 5.2% through 2030, reaching USD 1.8 billion.
- Increasing prevalence of dermatological and respiratory disorders, especially in aging and urban populations, underpin demand growth.
- Patent expiries and widespread generic manufacturing intensify price competition but also broaden access.
- Innovation in formulations (e.g., long-acting injectables, topical gels) remains crucial for differentiation.
- Growth in emerging markets, driven by healthcare development and affordability, offers significant upside.
FAQs
-
What are the primary therapeutic indications for triamcinolone?
Triamcinolone is used mainly for allergic reactions, skin conditions like eczema and psoriasis, nasal allergies, and certain inflammatory and autoimmune disorders.
-
How will patent expirations affect the triamcinolone market?
Patent expiries facilitate proliferation of generics, leading to price reductions but increasing market size due to improved affordability and accessibility.
-
What are the major challenges in marketing triamcinolone?
Potential side effects, regulatory scrutiny, and competition from other corticosteroids or biologics limit market growth and require strategic differentiation.
-
Which regions are expected to see the highest growth in triamcinolone sales?
The Asia-Pacific region is forecasted for the highest growth owing to expanding healthcare infrastructure, rising disease prevalence, and affordability.
-
Are biosimilars impacting the triamcinolone market?
While biosimilars are more common with biologic agents, generic corticosteroid formulations are influencing market dynamics by increasing competition and reducing prices.
Citations
[1] WHO. "Asthma Fact Sheet," 2020.
[2] Research and Markets. "Global Corticosteroids Market Analysis," 2021.
[3] Grand View Research. "Pharmaceuticals Market Size & Trends," 2022.