Last updated: July 27, 2025
Introduction
Risperidone, a second-generation antipsychotic medication developed by Janssen Pharmaceuticals, has established itself as a pivotal treatment option for schizophrenia, bipolar disorder, and irritability associated with autism. With a market that continues to evolve amid healthcare reforms, patent expirations, and emerging generics, understanding the current market dynamics and financial trajectory of risperidone is essential for stakeholders ranging from pharmaceutical companies to investors and healthcare providers.
This analysis explores the market landscape, key drivers, challenges, and future financial outlook for risperidone, providing nuanced insights into its strategic positioning within the pharmaceutical industry.
Market Overview and Demand Drivers
Global Market Size
The global antipsychotic drugs market, valued at approximately USD 16 billion in 2021, projected a compound annual growth rate (CAGR) of around 4% through 2028 [1]. Risperidone, accounting for a significant share, is a core product within this domain. The drug's primary indications—schizophrenia and bipolar disorder—drive sustained demand in North America, Europe, and emerging markets such as Asia-Pacific.
Clinical Efficacy and Safety Profile
Risperidone's reputation stems from its balanced efficacy and tolerability compared to first-generation antipsychotics; this has maintained its prominence in clinical practice. Its approval for multiple psychiatric conditions and formulations (oral and long-acting injectables) broaden its patient base.
Demographic and Epidemiological Factors
Increasing prevalence of schizophrenia and bipolar disorder, driven by urbanization, aging populations, and improved diagnostic criteria, sustains market demand. The World Health Organization estimates that schizophrenia affects over 20 million people globally, with treatment rates often exceeding 70% when accessible [2].
Market Dynamics: Key Drivers
Patent Expiration and Generics Competition
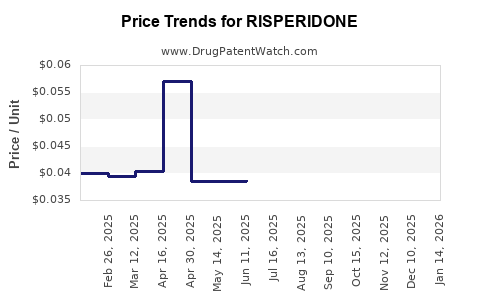
Risperidone's primary patent in the United States expired around 2008 for the oral formulation, leading to a surge in generic availability. However, the patent for the long-acting injectable (LAI) formulations, such as Risperdal Consta, persisted until 2018–2020. The entry of generics has significantly impacted Risperidone's market share and pricing strategies.
Pricing Pressure and Cost-Containment
Healthcare systems worldwide are under cost-containment pressures. Generics typically reduce treatment costs, leading to a decline in branded risperidone sales. However, branded formulations retain market segments, especially in regions with slower generic adoption or where physicians prefer proprietary products due to perceived efficacy or safety profiles.
Regulatory and Reimbursement Policies
Stringent regulatory approvals, varying reimbursement policies, and shifts towards value-based care influence risperidone's market penetration. Reimbursement favoring long-acting formulations in certain regions offsets some impact of generics by promoting new prescribing patterns.
Emergence of Newer Antipsychotics
The advent of third-generation antipsychotics such as aripiprazole and brexpiprazole offers alternative treatment options with potentially improved side-effect profiles. Their market share growth may cannibalize risperidone's demand in specific indications.
Innovations in Formulation and Delivery
Novel delivery systems, including depot injections with extended dosing intervals and digital adherence tools, enhance patient compliance and influence market dynamics. Janssen's development of longer-acting formulations aims to sustain competitive advantage.
Competitive Landscape
Key Players
- Janssen Pharmaceuticals: Original innovator with a diversified portfolio of risperidone formulations.
- Generics Manufacturers: Multiple players, including Teva, Sandoz, and Mylan, produce cost-effective generic equivalents widely adopted globally.
- New Entrants: Companies developing biosimilars or novel delivery methods challenge traditional formulations.
Market Share Distribution
Post-patent expiry, generics rapidly capturing market share diminished branded risperidone's dominance in developed nations. However, branded products maintain a significant presence in restricted markets and specific patient populations requiring tailored formulations.
Financial Trajectory Analysis
Revenue Trends
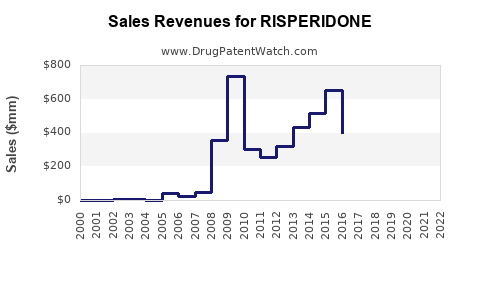
Following patent expiry, branded risperidone revenues in North America declined sharply, with estimates showing a 70% reduction within five years post-patent loss [3]. Nonetheless, in emerging markets, brand and generic sales continue to grow due to expanding healthcare access and high unmet needs.
Pricing and Profitability
Generic competition exerts downward pressure on unit prices. However, profit margins are preserved through volume growth, supply chain efficiencies, and higher-priced long-acting formulations. Innovation-driven premium pricing strategies are also employed in niche markets.
Research & Development Investments
Janssen's ongoing R&D efforts focus on new formulations and combination therapies, potentially creating additional revenue streams. Strategic licensing agreements for biosimilar or derivative products may influence future financial flows.
Forecasting the Future
Projections suggest that, despite generic erosion, risperidone's global revenues will stabilize around USD 1-2 billion annually through 2030, driven by emerging markets and formulation innovations [4].
Market Challenges and Opportunities
Challenges
- Market Saturation: Widespread generic adoption limits pricing power and margins.
- Regulatory Hurdles: Stringent approval pathways for new formulations and biosimilars.
- Patient Preferences: Growing demand for newer drugs with better side effect profiles.
Opportunities
- Extended-Release Formulations: Longer dosing intervals improve compliance and can command premium pricing.
- Combination Therapies: Co-formulations with other psychiatric agents present new market segments.
- Digital and Adherence Technologies: Telemedicine integrations and digital adherence tools support optimal therapy management.
Regulatory and Patent Outlook
While the original patents have lapsed, innovative formulations may qualify for new patents, securing exclusivity periods and enabling premium pricing. Regulatory pathways for biosimilars and digital health devices continue to shape competitive strategies.
Conclusion
Risperidone's market and financial landscape have undergone profound shifts driven by patent expirations, generics proliferation, and evolving treatment paradigms. Although traditional revenues declined post-patent, strategic focus on formulation innovation, emerging markets, and adjunct therapies presents growth avenues. Stakeholders must navigate complex regulatory and competitive environments to sustain profitability.
Key Takeaways
- Patent expirations triggered a significant decline in branded risperidone sales, replaced largely by generic formulations.
- Emerging markets and long-acting injectable formulations serve as growth catalysts amid generic competition.
- Innovation in delivery systems and combination therapies offer opportunities to extend risperidone’s market relevance.
- Pricing pressures necessitate value-based strategies, emphasizing patient adherence and clinical outcomes.
- Regulatory protections through new patents for formulations can sustain revenue streams and market exclusivity.
FAQs
1. How has patent expiration affected risperidone's market share?
Patent expiration, primarily for the oral formulation around 2008, led to a surge in generic competition, significantly reducing branded risperidone revenue and market share, especially in North America and Europe.
2. What are the primary factors influencing risperidone’s future sales?
Emerging formulations, market penetration in developing regions, competition from newer antipsychotics, and regulatory environments are key factors shaping future sales trajectories.
3. Are biosimilars impacting risperidone’s market?
Currently, biosimilars are not prevalent for risperidone, but future developments could introduce biosimilar versions, affecting pricing and market share.
4. What role does formulation innovation play in risperidone’s market strategy?
Innovations in long-acting depot injections and digital adherence tools help maintain competitive advantage and justify premium pricing despite generic pressure.
5. Which regions are expected to drive future growth for risperidone?
Emerging markets in Asia-Pacific, Latin America, and Africa, where healthcare infrastructure is expanding, are projected to underpin growth, alongside continued uptake of novel formulations globally.
References:
[1] Grand View Research, "Antipsychotic Drugs Market Size, Share & Trends Analysis," 2022.
[2] WHO, "Schizophrenia Fact Sheet," 2021.
[3] IQVIA, "Pharmaceutical Market Trends," 2022.
[4] MarketWatch, "Global Antipsychotic Market Forecast," 2022.