MERCK SHARP DOHME Company Profile
✉ Email this page to a colleague
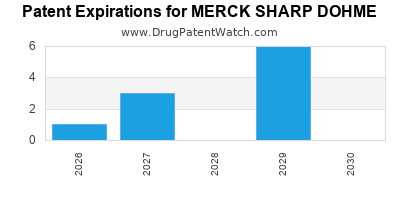
What is the competitive landscape for MERCK SHARP DOHME, and when can generic versions of MERCK SHARP DOHME drugs launch?
MERCK SHARP DOHME has twenty-three approved drugs.
There are twenty-five US patents protecting MERCK SHARP DOHME drugs.
There are six hundred and ninety patent family members on MERCK SHARP DOHME drugs in sixty-three countries and one hundred and twenty-seven supplementary protection certificates in nineteen countries.
Summary for MERCK SHARP DOHME
| International Patents: | 690 |
| US Patents: | 25 |
| Tradenames: | 19 |
| Ingredients: | 19 |
| NDAs: | 23 |
Drugs and US Patents for MERCK SHARP DOHME
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Merck Sharp Dohme | CLARINEX | desloratadine | SOLUTION;ORAL | 021300-001 | Sep 1, 2004 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Merck Sharp Dohme | TEMODAR | temozolomide | POWDER;INTRAVENOUS | 022277-001 | Feb 27, 2009 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Merck Sharp Dohme | PREVYMIS | letermovir | TABLET;ORAL | 209939-002 | Nov 8, 2017 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Merck Sharp Dohme | VICTRELIS | boceprevir | CAPSULE;ORAL | 202258-001 | May 13, 2011 | DISCN | No | No | 7,772,178 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Merck Sharp Dohme | VERQUVO | vericiguat | TABLET;ORAL | 214377-002 | Jan 19, 2021 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Merck Sharp Dohme | VERQUVO | vericiguat | TABLET;ORAL | 214377-003 | Jan 19, 2021 | RX | Yes | Yes | 8,420,656 | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for MERCK SHARP DOHME
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Merck Sharp Dohme | JUVISYNC | simvastatin; sitagliptin phosphate | TABLET;ORAL | 202343-004 | Sep 18, 2012 | 7,078,381 | ⤷ Try a Trial |
| Merck Sharp Dohme | PEPCID PRESERVATIVE FREE IN PLASTIC CONTAINER | famotidine | INJECTABLE;INJECTION | 020249-001 | Feb 18, 1994 | 4,283,408*PED | ⤷ Try a Trial |
| Merck Sharp Dohme | DUTREBIS | lamivudine; raltegravir potassium | TABLET;ORAL | 206510-001 | Feb 6, 2015 | 7,820,660 | ⤷ Try a Trial |
| Merck Sharp Dohme | TEMODAR | temozolomide | CAPSULE;ORAL | 021029-003 | Aug 11, 1999 | 5,260,291*PED | ⤷ Try a Trial |
| Merck Sharp Dohme | REBETOL | ribavirin | CAPSULE;ORAL | 020903-001 | Jun 3, 1998 | 6,472,373*PED | ⤷ Try a Trial |
| Merck Sharp Dohme | REBETOL | ribavirin | CAPSULE;ORAL | 020903-001 | Jun 3, 1998 | 6,461,605*PED | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for MERCK SHARP DOHME drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Capsules | 140 mg and 180 mg | ➤ Subscribe | 2008-03-24 |
| ➤ Subscribe | Delayed-release Tablets | 100 mg | ➤ Subscribe | 2014-06-16 |
| ➤ Subscribe | Tablets | 5 mg | ➤ Subscribe | 2006-06-21 |
| ➤ Subscribe | Tablets | 25 mg, 50 mg and 100 mg | ➤ Subscribe | 2010-10-18 |
| ➤ Subscribe | Tablets | 100 mg/10 mg and 100 mg/40 mg | ➤ Subscribe | 2012-06-25 |
| ➤ Subscribe | Oral Suspension | 40 mg/mL | ➤ Subscribe | 2011-02-28 |
| ➤ Subscribe | Injection | 18 mg/mL, 16.7 mL vials | ➤ Subscribe | 2015-11-24 |
| ➤ Subscribe | Orally Disintegrating Tablets | 2.5 mg and 5 mg | ➤ Subscribe | 2006-06-21 |
| ➤ Subscribe | Tablets | 100 mg/10 mg and 100 mg/40 mg | ➤ Subscribe | 2012-06-19 |
| ➤ Subscribe | Tablets | 50 mg/10 mg, 50 mg/20 mg, and 50 mg/40 mg | ➤ Subscribe | 2012-11-06 |
International Patents for MERCK SHARP DOHME Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Hungary | 0401730 | ⤷ Try a Trial |
| Slovenia | 3391890 | ⤷ Try a Trial |
| Slovenia | 2782914 | ⤷ Try a Trial |
| Lithuania | PA2008007 | ⤷ Try a Trial |
| Malaysia | 168412 | ⤷ Try a Trial |
| Norway | 2018022 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for MERCK SHARP DOHME Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0152897 | 2001C/013 | Belgium | ⤷ Try a Trial | PRODUCT NAME: DESLORATADINE; REGISTRTION NO/DATE: EU/1/00/160/010 20010115 |
| 1441735 | C20080001 00016 | Estonia | ⤷ Try a Trial | PRODUCT NAME: ISENTRESS; REG NO/DATE: 20.12.2007 C(2007)6801 |
| 1110543 | 08C0004 | France | ⤷ Try a Trial | PRODUCT NAME: DESLORATADINE; PSEUDOEPHEDRINE SULFATE; REGISTRATION NO/DATE: EU/1/07/399/001 20070730 |
| 1441735 | C 2008 008 | Romania | ⤷ Try a Trial | PRODUCT NAME: N-(2-(4-(4-FLUOROBENZILCARBAMOIL)-5-HIDROXI-1-METIL-6-OXO-1,6-DIHIDROPIRIMIDIN-2-IL)PROPAN-2+IL)-5-METIL-1,3,4-OXADIAZOL-2-CARBOXAMIDA - RALTEGRAVIR SAU O SARE ACCEPTABILAFARMACEUTIC A ACESTUIA, INSPECIAL SAREA DE POTASIU; NATIONAL AUTHORISATION NUMBER: EMEA EU/1/07/436/001, EMEA EU/1/07/436/002; DATE OF NATIONAL AUTHORISATION: 20071220; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EMEA EU/1/07/436/001, EMEA EU/1/07/436/002; DATE OF FIRST AUTHORISATION IN EEA: 20071220 |
| 1412357 | 2007/029 | Ireland | ⤷ Try a Trial | PRODUCT NAME: SITAGLIPTIN, OPTIONALLY IN THE FORM OF A PHARMACEUTICALLY ACCEPTABLE SALT; REGISTRATION NO/DATE: EU/1/07/383/001-018 20070321 |
| 1441735 | 2008/010 | Ireland | ⤷ Try a Trial | PRODUCT NAME: RALTEGRAVIR OR A PHARMECEUTICALLY ACCEPTABLE SALT THEREOF, ESPECIALLY THE POTASSIUM SALT; NAT AUTHORISTION NO/DATE: EU/1/07/436/001-002 20071220; |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.