PREVYMIS Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Prevymis, and when can generic versions of Prevymis launch?
Prevymis is a drug marketed by MSD and Merck Sharp Dohme and is included in three NDAs. There are two patents protecting this drug.
This drug has ninety-eight patent family members in forty-eight countries.
The generic ingredient in PREVYMIS is letermovir. One supplier is listed for this compound. Additional details are available on the letermovir profile page.
DrugPatentWatch® Generic Entry Outlook for Prevymis
Prevymis was eligible for patent challenges on November 8, 2021.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be August 30, 2031. This may change due to patent challenges or generic licensing.
There is one tentative approval for the generic drug (letermovir), which indicates the potential for near-term generic launch.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for PREVYMIS?
- What are the global sales for PREVYMIS?
- What is Average Wholesale Price for PREVYMIS?
Summary for PREVYMIS
| International Patents: | 98 |
| US Patents: | 2 |
| Applicants: | 2 |
| NDAs: | 3 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 48 |
| Clinical Trials: | 9 |
| Drug Prices: | Drug price information for PREVYMIS |
| What excipients (inactive ingredients) are in PREVYMIS? | PREVYMIS excipients list |
| DailyMed Link: | PREVYMIS at DailyMed |
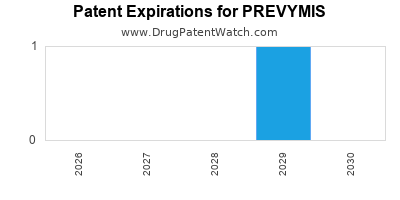

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for PREVYMIS
Generic Entry Dates for PREVYMIS*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
SOLUTION;INTRAVENOUS |
Generic Entry Dates for PREVYMIS*:
Constraining patent/regulatory exclusivity:
PROPHYLAXIS OF CYTOMEGALOVIRUS (CMV) DISEASE IN PEDIATRIC PATIENTS 12 YEARS OF AGE AND OLDER AND WEIGHING AT LEAST 40 KG WHO ARE KIDNEY TRANSPLANT RECIPIENTS AT HIGH RISK (DONOR CMV SEROPOSITIVE/RECIPIENT CMV SERONEGATIVE [D+/R-]) NDA:
Dosage:
TABLET;ORAL |
Generic Entry Dates for PREVYMIS*:
Constraining patent/regulatory exclusivity:
PROPHYLAXIS OF CYTOMEGALOVIRUS (CMV) DISEASE IN PEDIATRIC PATIENTS 12 YEARS OF AGE AND OLDER AND WEIGHING AT LEAST 40 KG WHO ARE KIDNEY TRANSPLANT RECIPIENTS AT HIGH RISK (DONOR CMV SEROPOSITIVE/RECIPIENT CMV SERONEGATIVE [D+/R-]) NDA:
Dosage:
PELLETS;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for PREVYMIS
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Merck Sharp & Dohme LLC | PHASE1 |
| Jason A Roberts | PHASE1 |
| Royal Brisbane and Women's Hospital | PHASE1 |
Pharmacology for PREVYMIS
US Patents and Regulatory Information for PREVYMIS
PREVYMIS is protected by two US patents and eight FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of PREVYMIS is ⤷ Get Started Free.
This potential generic entry date is based on PROPHYLAXIS OF CYTOMEGALOVIRUS (CMV) DISEASE IN PEDIATRIC PATIENTS 12 YEARS OF AGE AND OLDER AND WEIGHING AT LEAST 40 KG WHO ARE KIDNEY TRANSPLANT RECIPIENTS AT HIGH RISK (DONOR CMV SEROPOSITIVE/RECIPIENT CMV SERONEGATIVE [D+/R-]).
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Merck Sharp Dohme | PREVYMIS | letermovir | TABLET;ORAL | 209939-001 | Nov 8, 2017 | RX | Yes | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Merck Sharp Dohme | PREVYMIS | letermovir | TABLET;ORAL | 209939-001 | Nov 8, 2017 | RX | Yes | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Merck Sharp Dohme | PREVYMIS | letermovir | TABLET;ORAL | 209939-001 | Nov 8, 2017 | RX | Yes | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Msd | PREVYMIS | letermovir | PELLETS;ORAL | 219104-001 | Aug 30, 2024 | RX | Yes | No | ⤷ Get Started Free | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| Merck Sharp Dohme | PREVYMIS | letermovir | SOLUTION;INTRAVENOUS | 209940-002 | Nov 8, 2017 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Merck Sharp Dohme | PREVYMIS | letermovir | TABLET;ORAL | 209939-002 | Nov 8, 2017 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for PREVYMIS
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Merck Sharp Dohme | PREVYMIS | letermovir | SOLUTION;INTRAVENOUS | 209940-002 | Nov 8, 2017 | ⤷ Get Started Free | ⤷ Get Started Free |
| Merck Sharp Dohme | PREVYMIS | letermovir | SOLUTION;INTRAVENOUS | 209940-001 | Nov 8, 2017 | ⤷ Get Started Free | ⤷ Get Started Free |
| Merck Sharp Dohme | PREVYMIS | letermovir | TABLET;ORAL | 209939-002 | Nov 8, 2017 | ⤷ Get Started Free | ⤷ Get Started Free |
| Merck Sharp Dohme | PREVYMIS | letermovir | SOLUTION;INTRAVENOUS | 209940-001 | Nov 8, 2017 | ⤷ Get Started Free | ⤷ Get Started Free |
| Merck Sharp Dohme | PREVYMIS | letermovir | TABLET;ORAL | 209939-001 | Nov 8, 2017 | ⤷ Get Started Free | ⤷ Get Started Free |
| Merck Sharp Dohme | PREVYMIS | letermovir | TABLET;ORAL | 209939-002 | Nov 8, 2017 | ⤷ Get Started Free | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for PREVYMIS
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Merck Sharp & Dohme B.V. | Prevymis | letermovir | EMEA/H/C/004536Prevymis is indicated for prophylaxis of cytomegalovirus (CMV) reactivation and disease in adult CMV-seropositive recipients [R+] of an allogeneic haematopoietic stem cell transplant (HSCT).Consideration should be given to official guidance on the appropriate use of antiviral agents. | Authorised | no | no | yes | 2018-01-08 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for PREVYMIS
When does loss-of-exclusivity occur for PREVYMIS?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 13224947
Patent: Pharmaceutical preparation containing an antivirally active dihydroquinazoline derivative
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 2014020946
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 65203
Patent: PREPARATION PHARMACEUTIQUE CONTENANT UN DERIVE DE DIHYDROCHINAZOLINE A ACTION ANTIVIRALE (PHARMACEUTICAL COMPOSITION CONTAINING AN ANTIVIRALLY ACTIVE DIHYDROQUINAZOLINE DERIVATIVE)
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 14002306
Patent: Una composicion farmaceutica intravenosa que comprende al compuesto acido {8-fluoro-2-[4-(3-metoxifenil)-piperazina-1-il]-3-[2-metoxi-5-(trifluorometil)fenil]-3,4-dihidroquinazolina-4-il}acetico, al menos un excipiente seleccionado de ciclodextrinas, lisina y arginina, y agua; su metodo de preparacion; y su uso para el tratamiento y/o profilaxis de infecciones por virus.
Estimated Expiration: ⤷ Get Started Free
China
Patent: 4144678
Patent: Pharmaceutical preparation containing an antivirally active dihydroquinazoline derivative
Estimated Expiration: ⤷ Get Started Free
Patent: 0433166
Patent: 含有抗病毒活性二氢喹唑啉衍生物的药物制剂 (Pharmaceutical preparation containing antivirally active dihydroquinazoline derivative)
Estimated Expiration: ⤷ Get Started Free
Colombia
Patent: 61076
Patent: Composición farmacéutica que contiene un derivado de dihidroquinazolina antiviralmente activo
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0191369
Estimated Expiration: ⤷ Get Started Free
Patent: 0240197
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 21910
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 19648
Estimated Expiration: ⤷ Get Started Free
Patent: 56350
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 6584
Patent: ФАРМАЦЕВТИЧЕСКИЙ ПРЕПАРАТ, СОДЕРЖАЩИЙ ПРОИЗВОДНОЕ ДИГИДРОХИНАЗОЛИНА С ПРОТИВОВИРУСНОЙ АКТИВНОСТЬЮ (PHARMACEUTICAL PREPARATION CONTAINING AN ANTIVIRALLY ACTIVE DIHYDROQUINAZOLINE DERIVATIVE)
Estimated Expiration: ⤷ Get Started Free
Patent: 1400963
Patent: ФАРМАЦЕВТИЧЕСКИЙ ПРЕПАРАТ, СОДЕРЖАЩИЙ ПРОИЗВОДНОЕ ДИГИДРОХИНАЗОЛИНА С ПРОТИВОВИРУСНОЙ АКТИВНОСТЬЮ
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 19648
Patent: PRÉPARATION PHARMACEUTIQUE COMPRENANT UNE DÉRIVÉ ANTIVIRALE DE DIHYDROQUINAZOLINE (PHARMACEUTICAL PREPARATION COMPRISING AN ANTIVIRAL DIHYDROQUINAZOLINE DERIVATIVE)
Estimated Expiration: ⤷ Get Started Free
Patent: 56350
Patent: PRÉPARATION PHARMACEUTIQUE COMPRENANT UNE DÉRIVÉ ANTIVIRALE DE DIHYDROQUINAZOLINE AVEC UNE CONFIGURATION DE "S" A LA POSITION 4 (PHARMACEUTICAL PREPARATION COMPRISING AN ANTIVIRAL DIHYDROQUINAZOLINE DERIVATIVE WITH S CONFIGURATION IN POSITION 4)
Estimated Expiration: ⤷ Get Started Free
Patent: 28218
Patent: PRÉPARATION PHARMACEUTIQUE COMPRENANT UNE DÉRIVÉ ANTIVIRALE DE DIHYDROQUINAZOLINE (PHARMACEUTICAL PREPARATION COMPRISING AN ANTIVIRAL DIHYDROQUINAZOLINE DERIVATIVE)
Estimated Expiration: ⤷ Get Started Free
Finland
Patent: 56350
Estimated Expiration: ⤷ Get Started Free
Germany
Patent: 2012101680
Patent: Pharmazeutische Zubereitung enthaltend ein antiviral wirksames Dihydrochinazolinderivat
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 05462
Patent: 含有抗病毒活性二氫喹唑啉衍生物的藥物製劑 (PHARMACEUTICAL PREPARATION CONTAINING AN ANTIVIRALLY ACTIVE DIHYDROQUINAZOLINE DERIVATIVE)
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 45949
Estimated Expiration: ⤷ Get Started Free
Patent: 65553
Estimated Expiration: ⤷ Get Started Free
India
Patent: 92MUN2014
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 4363
Patent: תכשיר רוקחות המכיל נגזרת דיהידרוקוינאזולין עם פעילות אנטי-נגיפית (Pharmaceutical preparation containing an antivirally active dihydroquinazoline derivative)
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 87486
Estimated Expiration: ⤷ Get Started Free
Patent: 15508801
Patent: 抗ウイルス活性ジヒドロキナゾリン誘導体を含有する医薬組成物
Estimated Expiration: ⤷ Get Started Free
Lithuania
Patent: 19648
Estimated Expiration: ⤷ Get Started Free
Patent: 56350
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 2310
Patent: PHARMACEUTICAL PREPARATION CONTAINING AN ANTIVIRALLY ACTIVE DIHYDROQUINAZOLINE DERIVATIVE
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 9666
Patent: COMPOSICION FARMACEUTICA QUE CONTIENE UN DERIVADO DE DIHIDROQUINAZOLINA ANTIVIRALMENTE ACTIVO. (PHARMACEUTICAL PREPARATION CONTAINING AN ANTIVIRALLY ACTIVE DIHYDROQUINAZOLINE DERIVATIVE.)
Estimated Expiration: ⤷ Get Started Free
Patent: 14010364
Patent: COMPOSICION FARMACEUTICA QUE CONTIENE UN DERIVADO DE DIHIDROQUINAZOLINA ANTIVIRALMENTE ACTIVO. (PHARMACEUTICAL PREPARATION CONTAINING AN ANTIVIRALLY ACTIVE DIHYDROQUINAZOLINE DERIVATIVE.)
Estimated Expiration: ⤷ Get Started Free
Montenegro
Patent: 448
Patent: FARMACEUTSKI PREPARAT КОЈI OBUHVATA ANTIVIRUSNO EFIKASAN DERIVAT DIHIDROHINAZOLINA (PHARMACEUTICAL PREPARATION CONTAINING AN ANTIVIRALLY DIHYDROQUINAZOLINE DERIVATIVE)
Estimated Expiration: ⤷ Get Started Free
Morocco
Patent: 941
Patent: Préparation pharmaceutique contenant un dérivé de dihydrochinazoline à action antivirale
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 8444
Patent: Pharmaceutical preparation containing an antivirally active dihydroquinazoline derivative
Estimated Expiration: ⤷ Get Started Free
Philippines
Patent: 014501937
Patent: PHARMACEUTICAL COMPOSITION CONTAINING AN ANTIVIRALLY ACTIVE DIHYDROQUINAZOLINE DERIVATIVE
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 19648
Estimated Expiration: ⤷ Get Started Free
Patent: 56350
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 19648
Estimated Expiration: ⤷ Get Started Free
Patent: 56350
Estimated Expiration: ⤷ Get Started Free
San Marino
Patent: 01900458
Estimated Expiration: ⤷ Get Started Free
Patent: 02400068
Estimated Expiration: ⤷ Get Started Free
Serbia
Patent: 157
Patent: FARMACEUTSKI PREPARAT KOJI OBUHVATA ANTIVIRUSNO EFIKASAN DERIVAT DIHIDROHINAZOLINA (PHARMACEUTICAL PREPARATION COMPRISING AN ANTIVIRAL DIHYDROQUINAZOLINE DERIVATIVE)
Estimated Expiration: ⤷ Get Started Free
Patent: 137
Patent: FARMACEUTSKI PREPARAT KOJI OBUHVATA ANTIVIRUSNO EFIKASAN DERIVAT DIHIDROHINAZOLINA SA S KONFIGURACIJOM U POLOŽAJU 4 (PHARMACEUTICAL PREPARATION COMPRISING AN ANTIVIRAL DIHYDROQUINAZOLINE DERIVATIVE WITH S CONFIGURATION IN POSITION 4)
Estimated Expiration: ⤷ Get Started Free
Singapore
Patent: 201405294X
Patent: PHARMACEUTICAL PREPARATION CONTAINING AN ANTIVIRALLY ACTIVE DIHYDROQUINAZOLINE DERIVATIVE
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 19648
Estimated Expiration: ⤷ Get Started Free
Patent: 56350
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 1405949
Patent: PHARMACEUTICAL PREPARATION CONTAINING AND ANTIVIRALLY ACTIVE DIHYDROQUINAZOLINE DERIVATIVE
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 2149561
Estimated Expiration: ⤷ Get Started Free
Patent: 140130683
Patent: PHARMACEUTICAL PREPARATION CONTAINING AN ANTIVIRALLY ACTIVE DIHYDROQUINAZOLINE DERIVATIVE
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 41698
Estimated Expiration: ⤷ Get Started Free
Patent: 72133
Estimated Expiration: ⤷ Get Started Free
Tunisia
Patent: 14000345
Patent: PHARMACEUTICAL PREPARATION CONTAINING AN ANTIVIRALLY ACTIVE DIHYDROQUINAZOLINE DERIVATIVE
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 1415
Patent: ФАРМАЦЕВТИЧНИЙ ПРЕПАРАТ, ЩО МІСТИТЬ ПОХІДНУ ДИГІДРОХІНАЗОЛІНУ З ПРОТИВІРУСНОЮ АКТИВНІСТЮ (PHARMACEUTICAL PREPARATION CONTAINING DIHYDROQUINAZOLINE DERIVATIVE POSSESSING ANTIVIRAL ACTIVITY)
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering PREVYMIS around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| European Patent Office | 2819648 | PRÉPARATION PHARMACEUTIQUE COMPRENANT UNE DÉRIVÉ ANTIVIRALE DE DIHYDROQUINAZOLINE (PHARMACEUTICAL PREPARATION COMPRISING AN ANTIVIRAL DIHYDROQUINAZOLINE DERIVATIVE) | ⤷ Get Started Free |
| South Korea | 100927063 | ⤷ Get Started Free | |
| Israel | 234363 | תכשיר רוקחות המכיל נגזרת דיהידרוקוינאזולין עם פעילות אנטי-נגיפית (Pharmaceutical preparation containing an antivirally active dihydroquinazoline derivative) | ⤷ Get Started Free |
| Ukraine | 111415 | ФАРМАЦЕВТИЧНИЙ ПРЕПАРАТ, ЩО МІСТИТЬ ПОХІДНУ ДИГІДРОХІНАЗОЛІНУ З ПРОТИВІРУСНОЮ АКТИВНІСТЮ (PHARMACEUTICAL PREPARATION CONTAINING DIHYDROQUINAZOLINE DERIVATIVE POSSESSING ANTIVIRAL ACTIVITY) | ⤷ Get Started Free |
| Slovenia | 1622880 | ⤷ Get Started Free | |
| San Marino | T201900458 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for PREVYMIS
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1622880 | 300933 | Netherlands | ⤷ Get Started Free | PRODUCT NAME: LETERMOVIR; REGISTRATION NO/DATE: EU/1/17/1245 20180110 |
| 1622880 | 18C1029 | France | ⤷ Get Started Free | PRODUCT NAME: LETERMOVIR AINSI QUE SES SELS,SOLVATES ET SELS SOLVATES,PHARMACEUTIQUEMENT ACCEPTABLES; REGISTRATION NO/DATE: EU/1/17/1245/001-004 20180110 |
| 1622880 | CA 2018 00026 | Denmark | ⤷ Get Started Free | PRODUCT NAME: LETERMOVIR, OR ITS SALT, SOLVATE OR SOLVATE OF ITS SALT; REG. NO/DATE: EU/1/17/1245 20180110 |
| 1622880 | 122018000080 | Germany | ⤷ Get Started Free | PRODUCT NAME: LETERMOVIR ODER DESSEN SALZ, SOLVAT ODER SOLVAT DES SALZES; REGISTRATION NO/DATE: EU/1/17/1245 20180108 |
| 1622880 | C01622880/01 | Switzerland | ⤷ Get Started Free | FORMER OWNER: AICURIS ANTI-INFECTIVE CURES GMBH, DE |
| 1622880 | 697 | Finland | ⤷ Get Started Free | |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for PREVYMIS (Letermovir)
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
