Omeprazole - Generic Drug Details
✉ Email this page to a colleague
What are the generic drug sources for omeprazole and what is the scope of freedom to operate?
Omeprazole
is the generic ingredient in eight branded drugs marketed by Actavis Labs Fl Inc, Apotex, Aurobindo Pharma, Breckenridge, Dr Reddys Labs Ltd, Glenmark Pharms Ltd, Hetero Labs Ltd Iii, Impax Labs, Lannett Co Inc, Lupin Ltd, Pharmobedient, Sandoz, Strides Pharma, Teva Pharms Usa, Xiromed, Zydus Pharms Usa Inc, Astrazeneca, Dexcel Pharma, Dr Reddys, Sun Pharm, Dexcel, Aurobindo Pharma Ltd, L Perrigo Co, Spil, Covis, Marksans Pharma, P And L, Perrigo R And D, Ajanta Pharma Ltd, Anda Repository, Aurolife Pharma Llc, Chartwell Rx, Sciegen Pharms Inc, Strides Pharma Intl, Zydus, Zydus Pharms, Salix, Riley Consumer, Azurity, and Novitium Pharma, and is included in fifty-six NDAs. There are eleven patents protecting this compound and one Paragraph IV challenge. Additional information is available in the individual branded drug profile pages.Omeprazole has thirteen patent family members in eight countries.
There are one hundred and thirty-one drug master file entries for omeprazole. Ninety-three suppliers are listed for this compound. There is one tentative approval for this compound.
Summary for omeprazole
| International Patents: | 13 |
| US Patents: | 11 |
| Tradenames: | 8 |
| Applicants: | 40 |
| NDAs: | 56 |
| Drug Master File Entries: | 131 |
| Finished Product Suppliers / Packagers: | 93 |
| Raw Ingredient (Bulk) Api Vendors: | 144 |
| Clinical Trials: | 487 |
| Patent Applications: | 6,975 |
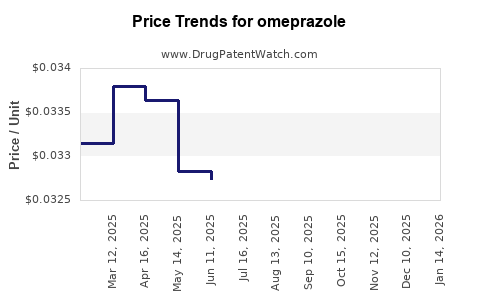
| Drug Prices: | Drug price trends for omeprazole |
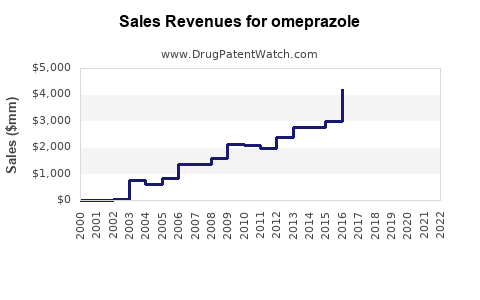
| Drug Sales Revenues: | Drug sales revenues for omeprazole |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for omeprazole |
| What excipients (inactive ingredients) are in omeprazole? | omeprazole excipients list |
| DailyMed Link: | omeprazole at DailyMed |
Recent Clinical Trials for omeprazole
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Rehman Medical Institute - RMI | PHASE4 |
| Bristol-Myers Squibb | PHASE1 |
| Genentech, Inc. | PHASE1 |
Generic filers with tentative approvals for OMEPRAZOLE
| Applicant | Application No. | Strength | Dosage Form |
| ⤷ Get Started Free | ⤷ Get Started Free | 40MG | CAPSULE, DELAYED REL PELLETS;ORAL |
| ⤷ Get Started Free | ⤷ Get Started Free | 20MG | CAPSULE, DELAYED REL PELLETS;ORAL |
| ⤷ Get Started Free | ⤷ Get Started Free | 10MG | CAPSULE, DELAYED REL PELLETS;ORAL |
The 'tentative' approval signifies that the product meets all FDA standards for marketing, and, but for the patents / regulatory protections, it would approved.
Pharmacology for omeprazole
| Drug Class | Proton Pump Inhibitor |
| Mechanism of Action | Cytochrome P450 2C19 Inhibitors Proton Pump Inhibitors |
Medical Subject Heading (MeSH) Categories for omeprazole
Anatomical Therapeutic Chemical (ATC) Classes for omeprazole
Paragraph IV (Patent) Challenges for OMEPRAZOLE
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| OMEPRAZOLE | Delayed-release Tablets | omeprazole | 20 mg | 022032 | 1 | 2015-06-03 |
US Patents and Regulatory Information for omeprazole
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ajanta Pharma Ltd | OMEPRAZOLE AND SODIUM BICARBONATE | omeprazole; sodium bicarbonate | FOR SUSPENSION;ORAL | 205545-002 | Jul 27, 2016 | AB | RX | No | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Impax Labs | OMEPRAZOLE | omeprazole | CAPSULE, DELAYED REL PELLETS;ORAL | 075785-003 | Jan 21, 2009 | AB | RX | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Astrazeneca | PRILOSEC | omeprazole | CAPSULE, DELAYED REL PELLETS;ORAL | 019810-003 | Oct 5, 1995 | DISCN | Yes | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for omeprazole
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Astrazeneca | PRILOSEC | omeprazole | CAPSULE, DELAYED REL PELLETS;ORAL | 019810-001 | Sep 14, 1989 | 4,636,499*PED | ⤷ Get Started Free |
| Astrazeneca | PRILOSEC | omeprazole | CAPSULE, DELAYED REL PELLETS;ORAL | 019810-001 | Sep 14, 1989 | 6,166,213*PED | ⤷ Get Started Free |
| Astrazeneca | PRILOSEC | omeprazole | CAPSULE, DELAYED REL PELLETS;ORAL | 019810-002 | Jan 15, 1998 | 6,191,148*PED | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for omeprazole
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| World Intellectual Property Organization (WIPO) | 0078284 | ⤷ Get Started Free | |
| European Patent Office | 3471708 | ⤷ Get Started Free | |
| Spain | 2304349 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for omeprazole
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1411900 | 2011/016 | Ireland | ⤷ Get Started Free | PRODUCT NAME: NAPROXEN AND ESOMEPRAZOLE MODIFIED-RELEASE TABLETS; NAT REGISTRATION NO/DATE: PA0970/060/001 20101221; FIRST REGISTRATION NO/DATE: PL17901/0263-0001 20101105 |
| 1411900 | 2011C/016 | Belgium | ⤷ Get Started Free | PRODUCT NAME: NAPROXENE ET ESOMEPRAZOLE (SOUS LA FORME D'ESOMEPRAZOLE MAGNESIUM TRIHYDRATE); AUTHORISATION NUMBER AND DATE: BE382505 20101214 |
| 0124495 | SPC/GB01/006 | United Kingdom | ⤷ Get Started Free | PRODUCT NAME: ESOMEPRAZOLE AS MAGNESIUM TRIHYDRATE; REGISTERED: SE 15945 20000310; SE 15946 20000310; UK PL 17901/0068-0069 20000727 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for Omeprazole
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.