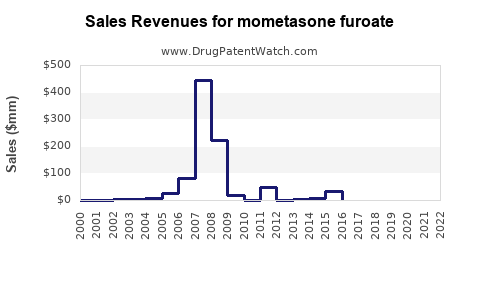
Mometasone furoate - Generic Drug Details
✉ Email this page to a colleague
What are the generic sources for mometasone furoate and what is the scope of patent protection?
Mometasone furoate
is the generic ingredient in eight branded drugs marketed by Organon Llc, Organon, Anda Repository, Cosette, Fougera Pharms, Glenmark Pharms Inc, Taro, Intersect Ent Inc, Encube, Glenmark Pharms Ltd, Padagis Israel, Padagis Us, Torrent, Amneal, Amneal Pharms, Apotex, Aurobindo Pharma, Perrigo Pharma Intl, and Glenmark Speclt, and is included in thirty-two NDAs. There are twenty-eight patents protecting this compound and two Paragraph IV challenges. Additional information is available in the individual branded drug profile pages.Mometasone furoate has forty-seven patent family members in twelve countries.
There are twenty-seven drug master file entries for mometasone furoate. Twenty-three suppliers are listed for this compound. There is one tentative approval for this compound.
Summary for mometasone furoate
| International Patents: | 47 |
| US Patents: | 28 |
| Tradenames: | 8 |
| Applicants: | 19 |
| NDAs: | 32 |
| Drug Master File Entries: | 27 |
| Finished Product Suppliers / Packagers: | 23 |
| Raw Ingredient (Bulk) Api Vendors: | 79 |
| Clinical Trials: | 143 |
| Patent Applications: | 6,223 |
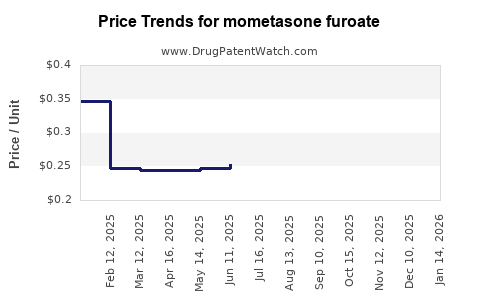
| Drug Prices: | Drug price trends for mometasone furoate |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for mometasone furoate |
| What excipients (inactive ingredients) are in mometasone furoate? | mometasone furoate excipients list |
| DailyMed Link: | mometasone furoate at DailyMed |
Recent Clinical Trials for mometasone furoate
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Zheng Liu | N/A |
| MEDA Pharma GmbH & Co. KG | Phase 2 |
| Morten Hostrup, PhD | N/A |
Generic filers with tentative approvals for MOMETASONE FUROATE
The 'tentative' approval signifies that the product meets all FDA standards for marketing, and, but for the patents / regulatory protections, it would approved.
Pharmacology for mometasone furoate
| Drug Class | Corticosteroid |
| Mechanism of Action | Corticosteroid Hormone Receptor Agonists |
Medical Subject Heading (MeSH) Categories for mometasone furoate
Anatomical Therapeutic Chemical (ATC) Classes for mometasone furoate
Paragraph IV (Patent) Challenges for MOMETASONE FUROATE
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| NASONEX | Nasal Spray | mometasone furoate | 50 mcg/ Spray | 020762 | 1 | 2009-08-07 |
| ELOCON | Topical Solution (Lotion) | mometasone furoate | 0.1% | 019796 | 1 | 2004-06-10 |
US Patents and Regulatory Information for mometasone furoate
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intersect Ent Inc | SINUVA | mometasone furoate | IMPLANT;IMPLANTATION | 209310-001 | Dec 8, 2017 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Glenmark Speclt | RYALTRIS | mometasone furoate; olopatadine hydrochloride | SPRAY, METERED;NASAL | 211746-001 | Jan 13, 2022 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Amneal Pharms | MOMETASONE FUROATE | mometasone furoate | SPRAY, METERED;NASAL | 207989-001 | Apr 3, 2017 | AB | RX | No | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| Perrigo Pharma Intl | NASONEX 24HR ALLERGY | mometasone furoate | SPRAY, METERED;NASAL | 215712-001 | Mar 17, 2022 | OTC | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Aurobindo Pharma | MOMETASONE FUROATE | mometasone furoate | SPRAY, METERED;NASAL | 215878-001 | Mar 18, 2024 | AB | RX | No | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for mometasone furoate
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Organon Llc | ASMANEX HFA | mometasone furoate | AEROSOL, METERED;INHALATION | 205641-002 | Apr 25, 2014 | ⤷ Sign Up | ⤷ Sign Up |
| Organon Llc | ASMANEX TWISTHALER | mometasone furoate | POWDER;INHALATION | 021067-002 | Feb 1, 2008 | ⤷ Sign Up | ⤷ Sign Up |
| Organon Llc | ASMANEX TWISTHALER | mometasone furoate | POWDER;INHALATION | 021067-001 | Mar 30, 2005 | ⤷ Sign Up | ⤷ Sign Up |
| Organon Llc | ASMANEX TWISTHALER | mometasone furoate | POWDER;INHALATION | 021067-001 | Mar 30, 2005 | ⤷ Sign Up | ⤷ Sign Up |
| Organon Llc | NASONEX | mometasone furoate | SPRAY, METERED;NASAL | 020762-001 | Oct 1, 1997 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for mometasone furoate
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Singapore | 11201507476T | SYSTEMS, DEVICES, AND METHOD FOR TREATING A SINUS CONDITION | ⤷ Sign Up |
| European Patent Office | 3103422 | DISTRIBUTION PAR SINUS D'AGENTS THÉRAPEUTIQUES À LIBÉRATION PROLONGÉE (SINUS DELIVERY OF SUSTAINED RELEASE THERAPEUTICS) | ⤷ Sign Up |
| China | 102573981 | Expandable devices and methods therefor | ⤷ Sign Up |
| China | 105188831 | Systems, devices, and methods for treating a sinus condition | ⤷ Sign Up |
| Japan | 5247428 | ⤷ Sign Up | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for mometasone furoate
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 3043773 | SPC/GB21/077 | United Kingdom | ⤷ Sign Up | PRODUCT NAME: MOMETASONE OR A SALT THEREOF AND OLOPATADINE OR A SALT THEREOF; REGISTERED: AT 140638 20210426; UK PL 25258/0331 - 0001 20210511 |
| 3043773 | 21C1057 | France | ⤷ Sign Up | PRODUCT NAME: MOMETASONE OU L'UN DE SES SELS AVEC OLOPATADINE OU L'UN DE SES SELS; NAT. REGISTRATION NO/DATE: NL52121 20211026; FIRST REGISTRATION: AT - 140638 20210426 |
| 3043773 | 2190041-0 | Sweden | ⤷ Sign Up | PRODUCT NAME: MOMETASONE OR A SALT THEREOF AND OLOPATADINE OR A SALT THEREOF; NAT. REG. NO/DATE: MT NR 60226 20210519; FIRST REG.: AT APPROVAL NR 140638 20210426 |
| 0548114 | SPC/GB97/064 | United Kingdom | ⤷ Sign Up | PRODUCT NAME: MOMETASONE FUROATE MONOHYDRATE; REGISTERED: FR AMM 343012.6 19970219; UK 00201/0216 19970410 |
| 3043773 | 2022C/520 | Belgium | ⤷ Sign Up | PRODUCT NAME: MOMETASONE OF EEN ZOUT HIERVAN EN OLOPATADINE OF EEN ZOUT HIERVAN; AUTHORISATION NUMBER AND DATE: BE595626 20220203 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.