GILEAD SCIENCES INC Company Profile
✉ Email this page to a colleague
What is the competitive landscape for GILEAD SCIENCES INC, and what generic alternatives to GILEAD SCIENCES INC drugs are available?
GILEAD SCIENCES INC has twenty-three approved drugs.
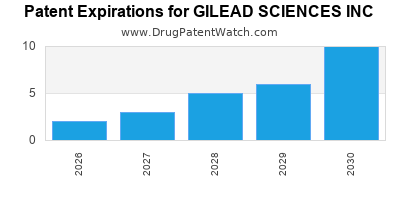
There are seventy-nine US patents protecting GILEAD SCIENCES INC drugs.
There are two thousand two hundred and twenty-eight patent family members on GILEAD SCIENCES INC drugs in sixty-six countries and three hundred and seventy supplementary protection certificates in nineteen countries.
Summary for GILEAD SCIENCES INC
| International Patents: | 2228 |
| US Patents: | 79 |
| Tradenames: | 18 |
| Ingredients: | 18 |
| NDAs: | 23 |
| Drug Master File Entries: | 7 |
| Patent Litigation for GILEAD SCIENCES INC: | See patent lawsuits for GILEAD SCIENCES INC |
Drugs and US Patents for GILEAD SCIENCES INC
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gilead Sciences Inc | VEKLURY | remdesivir | POWDER;INTRAVENOUS | 214787-001 | Oct 22, 2020 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Gilead Sciences Inc | COMPLERA | emtricitabine; rilpivirine hydrochloride; tenofovir disoproxil fumarate | TABLET;ORAL | 202123-001 | Aug 10, 2011 | RX | Yes | Yes | 9,457,036 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Gilead Sciences Inc | VOSEVI | sofosbuvir; velpatasvir; voxilaprevir | TABLET;ORAL | 209195-001 | Jul 18, 2017 | RX | Yes | Yes | 11,338,007*PED | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Gilead Sciences Inc | HARVONI | ledipasvir; sofosbuvir | PELLETS;ORAL | 212477-001 | Aug 28, 2019 | RX | Yes | No | 8,633,309*PED | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for GILEAD SCIENCES INC
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Gilead Sciences Inc | ODEFSEY | emtricitabine; rilpivirine hydrochloride; tenofovir alafenamide fumarate | TABLET;ORAL | 208351-001 | Mar 1, 2016 | 5,814,639*PED | ⤷ Try a Trial |
| Gilead Sciences Inc | COMPLERA | emtricitabine; rilpivirine hydrochloride; tenofovir disoproxil fumarate | TABLET;ORAL | 202123-001 | Aug 10, 2011 | 6,838,464 | ⤷ Try a Trial |
| Gilead Sciences Inc | VIREAD | tenofovir disoproxil fumarate | TABLET;ORAL | 021356-003 | Jan 18, 2012 | 6,043,230*PED | ⤷ Try a Trial |
| Gilead Sciences Inc | STRIBILD | cobicistat; elvitegravir; emtricitabine; tenofovir disoproxil fumarate | TABLET;ORAL | 203100-001 | Aug 27, 2012 | 8,592,397 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for GILEAD SCIENCES INC drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Tablets | 150 mg, 200 mg, and 250 mg | ➤ Subscribe | 2012-05-17 |
| ➤ Subscribe | Tablets | 150 mg, 150 mg, 200 mg, 300 mg | ➤ Subscribe | 2018-10-04 |
| ➤ Subscribe | Tablets | 300 mg | ➤ Subscribe | 2010-01-26 |
| ➤ Subscribe | Tablets | 200 mg/25 mg/300 mg | ➤ Subscribe | 2015-05-20 |
| ➤ Subscribe | Tablets | 150 mg | ➤ Subscribe | 2015-12-09 |
International Patents for GILEAD SCIENCES INC Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| European Patent Office | 3626716 | ⤷ Try a Trial |
| South Korea | 102337664 | ⤷ Try a Trial |
| Brazil | 112014011938 | ⤷ Try a Trial |
| Slovenia | 2873665 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for GILEAD SCIENCES INC Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2487163 | 300859 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: COBICISTAT, DAN WEL EEN FARMACEUTISCH AANVAARDBAAR ZOUT DAARVAN EN ATAZANAVIR, DAN WEL EEN FARMACEUTISCH AANVAARDBAAR ZOUT DAARVAN, IN HET BIJZONDER ATAZANAVIRSULFAAT; REGISTRATION NO/DATE: EU/1/15/1025 20150715 |
| 1419152 | SPC/GB12/022 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: RILPIVIRINE, A N-OXIDE, A PHARMACEUTICALLY ACCEPTABLE ADDITION SALT INCLUDING THE HYDROCHLORIC ACID SALT, OR A QUATERNARY AMINE THEREOF.; REGISTERED: UK EU/1/11/736/001 20111128 |
| 2487166 | 2016C/065 | Belgium | ⤷ Try a Trial | PRODUCT NAME: COBICISTAT AND TENOFOVIR ALAFENAMIDE; AUTHORISATION NUMBER AND DATE: EU/1/15/1061 20151123 |
| 1564210 | 92307 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: ELVITEGRAVIR SOUS TOUTES SES FORMES COMME PROTEGEES PAR LE BREVET DE BASE; AUTHORISATION NUMBER AND DATE: EU/1/13/830/001-002 - STRIBILD-ELVITEGRAVIR/... 20130527 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.