The pharmaceutical industry stands at a fascinating juncture, where the pursuit of affordable healthcare intersects with the pinnacle of scientific innovation. Generic drugs, often perceived as mere copies of their brand-name counterparts, are, in reality, products of sophisticated scientific endeavor and strategic foresight. They represent a critical pillar of global healthcare, driving down costs and expanding patient access to essential medicines. Yet, the journey from patent expiry to market entry is fraught with complex formulation challenges that demand ingenuity, advanced technology, and a meticulous understanding of both drug science and regulatory frameworks.
This report delves into the intricate world of generic drug formulation, dissecting the core challenges, exploring pioneering solutions, and illuminating the strategic advantages available to companies that master this demanding domain. We will navigate the physicochemical complexities of matching reference products, the nuances of specialized dosage forms, and the critical role of intellectual property and competitive intelligence in shaping market success.
The Strategic Imperative of Generic Drug Development
Generic drugs are not just cheaper alternatives; they are fundamental to the economic viability and accessibility of healthcare systems worldwide. Their development and market entry are driven by a compelling blend of public health necessity and astute business strategy.
Generic Drugs: Pillars of Affordable Healthcare
The very foundation of generic drug development rests on the principle of therapeutic equivalence, a concept that underpins patient trust and market acceptance.
Defining Generic Drugs and Bioequivalence: The Cornerstone of Trust
At its heart, a generic drug is a pharmaceutical product containing the same active chemical substance as a proprietary drug that was once protected by patents. These drugs are permitted for sale once the original patents expire . While the active pharmaceutical ingredient (API) must be identical to the original, generic drugs are allowed to differ in various characteristics such as the manufacturing process, formulation, excipients (inactive ingredients), color, taste, and packaging . This flexibility, however, introduces a profound scientific challenge.
The U.S. Food and Drug Administration (FDA) mandates that generics be identical to, or fall within an acceptable bioequivalent range of, their brand-name counterparts, specifically concerning pharmacokinetic (PK) and pharmacodynamic (PD) properties . What does “bioequivalent” truly mean in practice? It signifies that the generic version must release its active ingredient into the bloodstream at virtually the same speed and in virtually the same amounts as the original drug . This is typically demonstrated through rigorous in vivo studies in human subjects, where key PK parameters like peak drug plasma concentration (Cmax) and the area under the drug plasma concentration versus time profile (AUC) are characterized and compared. For a generic to be deemed bioequivalent, the 90% confidence interval of the geometric mean test/reference ratios for both AUC and Cmax must fall within the limits of 80–125% .
Consider the subtle, yet significant, distinction here: while the public often assumes generic drugs are exact chemical clones, the regulatory definition of “bioequivalence” allows for variations. For instance, a different salt or ester of the active ingredient might be used, or different inactive ingredients might be incorporated . This creates a unique challenge for generic developers, often referred to as the “invisible” equivalence challenge. They are not merely copying a formula; they are reverse-engineering a performance profile. This demands an intricate understanding of how changes in formulation components, even seemingly inert ones, can influence the drug’s absorption, distribution, metabolism, and excretion (ADME) within the human body. It’s a delicate dance of achieving identical therapeutic outcomes through potentially different compositional pathways.
The Economic and Societal Impact of Generic Entry
The widespread adoption of generic drugs has fundamentally reshaped global healthcare economics, delivering monumental cost savings and enhancing patient access.
The economic impact is staggering. A single generic competitor entering the market can lead to price reductions of 30%, while the presence of five competing generics can drive prices down by nearly 85% . This competitive dynamic has translated into immense savings for healthcare systems and patients alike. From 2009 to 2019, generic drugs saved the U.S. healthcare system an estimated $2.2 trillion . More recently, in 2022 alone, new generic approvals generated approximately $53.3 billion in savings .
The global generic drugs market is a testament to this economic power. Valued at USD 445.62 billion in 2024, it is projected to grow to approximately USD 728.64 billion by 2034, demonstrating a Compound Annual Growth Rate (CAGR) of 5.04% . This robust growth is primarily fueled by the inherently lower cost of generics compared to branded drugs and the continuous expiration of patents on blockbuster medications .
Beyond the raw numbers, the societal benefits are profound. Generics significantly improve patient access to essential medications, particularly benefiting low-income populations and developing countries where affordability is a critical barrier . Moreover, the introduction of multiple generic manufacturers for a single drug product helps stabilize the supply chain and mitigates the risk of drug shortages, a growing concern in today’s complex global environment .
However, this picture of success is not without its complexities. The very mechanism that drives down drug prices—intense competition—can paradoxically strain the generic manufacturing sector. The pursuit of aggressive price reductions often leads to razor-thin profit margins in conventional generic segments . This can create supply chain vulnerabilities and even compromise quality if manufacturers are forced to cut corners to remain competitive . This delicate balance between maximizing cost savings for consumers and ensuring the sustainable profitability and quality of generic manufacturers is a critical challenge that demands continuous strategic innovation within the industry.
To underscore the profound economic influence of generic entry, consider the typical price trajectory following patent expiration:
| Number of Generic Competitors | Approximate Price Reduction vs. Brand |
| 1 | 30% |
| 3 | 20% |
| 5 | 85% |
| 10+ | 70-80% |
This table vividly illustrates how competition acts as a powerful economic force, driving down prices and delivering substantial value to healthcare systems and patients.
Beyond Replication: The Evolving Landscape of Generic Formulation
The journey of a generic drug is far more intricate than simply copying a formula; it’s an evolving scientific and business challenge that demands innovation.
Why Generic Formulation is a Distinct Scientific and Business Challenge
Unlike the development of an innovator drug, which necessitates extensive and costly clinical trials to prove safety and efficacy from scratch, generic drug development operates on an abbreviated pathway. The U.S. FDA’s Abbreviated New Drug Application (ANDA) process and the European Medicines Agency’s (EMA) Marketing Authorisation Application (MAA) for generics allow manufacturers to bypass these lengthy clinical trials because the safety and efficacy of the active ingredient have already been established by the Reference Listed Drug (RLD) .
Instead, the core challenge for generic developers lies in demonstrating therapeutic equivalence through rigorous bioequivalence (BE) studies . This means proving that the generic product delivers the same active substance, at the same rate and extent, as the innovator drug . But here’s the rub: achieving this “sameness” is anything but simple. Generic formulations are permitted to differ from the RLD in their inactive ingredients, manufacturing processes, and even the specific salt or ester forms of the API . These seemingly minor differences can, in fact, profoundly impact a drug’s performance, stability, and bioavailability in the human body .
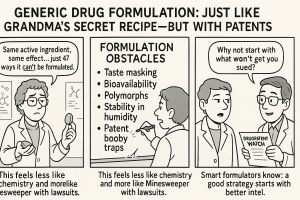
This leads to a unique predicament for generic developers: they must replicate the performance of an RLD without having full access to its proprietary formulation details or manufacturing blueprints. This is often described as the challenge of “known unknowns” in RLD formulation. It necessitates extensive reverse-engineering, a process often termed “de-formulation,” to meticulously understand the RLD’s critical quality attributes (CQAs) and precisely how they are achieved . It’s akin to a master chef trying to replicate a secret recipe by tasting the dish and analyzing its texture, rather than having the ingredient list and cooking instructions. This transforms generic development from a simple copying exercise into a sophisticated scientific and engineering challenge, demanding deep analytical capabilities and innovative formulation expertise.
The Shift Towards Complex Generics: A New Frontier
The generic pharmaceutical market is not static; it is constantly evolving, driven by both scientific advancement and economic pressures. A significant trend observed in recent years is the industry’s strategic pivot towards “complex generics.”
Why this shift? The market for simpler generic drugs has become increasingly saturated, leading to intense competition and, as discussed, razor-thin profit margins . To sustain growth and profitability, pharmaceutical companies are increasingly focusing on these more challenging, yet potentially more lucrative, complex generic products . These products are inherently more difficult to develop, often resulting in fewer generic competitors and thus greater market potential .
Complex generics are a diverse category, encompassing products with:
- Complex Active Pharmaceutical Ingredients (APIs): This includes intricate molecules like peptides, complex mixtures, or those derived from natural sources .
- Complex Formulations: Examples include liposomes, nanoemulsions, protein-drug complexes, and iron colloids, which require comprehensive physicochemical characterization to ensure equivalence .
- Complex Dosage Forms or Routes of Delivery: This covers a wide range, from modified-release (MR) formulations designed for sustained or delayed drug release, to transdermal patches, and even locally acting drugs where systemic absorption is not the primary goal .
- Drug-Device Combinations: Products like metered-dose inhalers (pMDIs), dry powder inhalers (DPIs), and auto-injectors, where the drug formulation and the delivery device are inextricably linked and both must be equivalent to the RLD .
The development of these complex generics requires specific studies that go far beyond those needed for simple generics to demonstrate therapeutic equivalence . This often translates into substantial investment and a significant commitment of resources from manufacturers . For generic companies, this move into complex generics is not merely a scientific progression; it represents a fundamental strategic imperative. It’s a calculated move to escape the commoditization and intense price competition of simpler segments. This shift necessitates significant R&D investment, the adoption of novel formulation approaches, and a willingness to push the boundaries of pharmaceutical science. In essence, it is transforming generic companies from pure cost-cutters into innovators in their own right, capable of developing differentiated products that offer enhanced therapeutic profiles and address unmet patient needs.
Unpacking the Core Formulation Challenges
The journey to replicate a brand-name drug’s performance is paved with scientific and technical hurdles. Let us dissect the core formulation challenges that generic developers must meticulously overcome.
The Physicochemical Conundrum: Matching the Reference Listed Drug (RLD)
At the heart of generic drug development lies the intricate task of matching the physicochemical properties of the RLD, a challenge that extends beyond simple chemical identity.
Q1/Q2/Q3 Sameness: The Quest for Equivalence
The regulatory framework for generic drugs often hinges on the concept of “sameness,” particularly concerning inactive ingredients. This is encapsulated in the terms “Q1/Q2 sameness” and “Q3 sameness.”
Q1 (Qualitative) sameness dictates that a proposed generic formulation must contain the same inactive ingredients as its RLD. To demonstrate this, applicants are required to provide detailed information on the chemistry of each inactive ingredient, along with characterization data if necessary . This isn’t just a checklist; it demands a thorough understanding of the excipient’s identity and purity.
Q2 (Quantitative) sameness takes this a step further, requiring that the concentrations of these inactive ingredients in the generic product be the same as those used in the RLD. The FDA generally interprets “Q2 sameness” to mean that the concentration must be within 95-105% of the RLD concentration .
Beyond the chemical composition of inactive ingredients, Q3 sameness extends the equivalence requirement to the physical and structural characteristics of the drug product. This means the generic must exhibit similar physical and structural properties to the reference product, encompassing aspects like particle morphology, surface potential, and size distribution .
For certain dosage forms, such as parenteral (injectable), ophthalmic (eye), and otic (ear) products, Q1/Q2 sameness is often a regulatory requirement . However, even here, minor differences in components like preservatives, buffers, or antioxidants may be allowed, provided the applicant can demonstrate that these differences do not affect the safety or efficacy of the proposed drug product . For other routes of administration where Q1/Q2 sameness is not strictly mandated, a non-Q1/Q2 application may be submitted, although product-specific guidances (PSGs) might then recommend different bioequivalence (BE) approaches .
This intricate web of Q1/Q2/Q3 requirements illustrates a critical tension in generic drug development: regulators demand “sameness” to ensure safety and efficacy, yet they also allow for “permissible differences” to foster generic competition. Navigating this regulatory tightrope requires not only deep scientific understanding but also strategic regulatory engagement. Deviations, even seemingly minor ones, can trigger extensive additional studies, leading to significant delays or even outright rejection of an Abbreviated New Drug Application (ANDA) . The challenge lies in proving that any deviation in inactive ingredients or manufacturing processes does not compromise the drug’s performance, a task that often demands sophisticated analytical and bioequivalence testing.
Solubility and Dissolution: The Gateway to Bioavailability
For a drug to exert its therapeutic effect, it must first be absorbed into the bloodstream. For many oral medications, this journey begins with dissolution, and herein lies a significant hurdle for generic developers.
Low aqueous solubility is a pervasive problem in pharmaceutical formulation, affecting both novel chemical entities (NCEs) and generic drugs. It is estimated that more than 40% of NCEs developed in the pharmaceutical industry are practically insoluble in water . Why is this a problem? Because for any drug to be absorbed effectively, it must first be present in a dissolved form at the site of absorption . Factors contributing to poor solubility often include a molecule’s high melting point (MP) and high partition coefficient (logP), which indicate a preference for organic phases over aqueous ones . Poor solubility directly limits the extent to which a drug can be absorbed, thereby impacting its bioavailability.
Dissolution testing emerges as a fundamental analytical technique in this context. It serves as a crucial predictor of drug release and bioavailability, ensuring consistency in drug release and meeting stringent regulatory expectations for both innovator and generic products . For poorly water-soluble drugs, achieving “sink conditions”—where the drug dissolves readily into the dissolution medium—can be particularly challenging. In such cases, the incorporation of suitable surfactants into the dissolution medium is often necessary to facilitate the process .
To overcome these solubility and dissolution challenges, generic developers employ a range of enhancement techniques:
- Particle Size Reduction: Techniques like micronization and nano-milling are commonly used in the preformulation stage to increase the dissolution rate of APIs whose absorption is limited by their particle size (classified as BCS Class IIb drugs) . By increasing the surface area, the drug can dissolve more rapidly.
- Amorphous Solid Dispersion (ASD) and Lipid-Based Formulations: For compounds whose absorption is limited by their intrinsic solubility (BCS Class IIa), ASD technologies and lipid-based formulations have become primary approaches. ASD involves dispersing drug molecules within a water-soluble polymer matrix, significantly improving dissolution rates and bioavailability .
- Other Conventional Approaches: These include pH adjustment, salt formation to improve solubility, complexation (e.g., with cyclodextrins), and the strategic use of surfactants .
The Biopharmaceutics Classification System (BCS) is not merely an academic classification; it serves as a strategic compass for generic drug development. By categorizing drugs based on their solubility and permeability, the BCS framework directly guides the appropriate dissolution strategies and, crucially, determines whether a biowaiver for in vivo bioequivalence studies is feasible . For instance, BCS Class 1 drugs (high solubility, high permeability) and certain BCS Class 3 drugs (high solubility, low permeability) may be eligible for biowaivers, which can significantly reduce the need for costly and time-consuming in vivo studies, thereby streamlining development timelines and reducing overall costs . Understanding and leveraging a drug’s BCS classification is therefore a pivotal strategic decision in generic formulation, directly impacting the economic and temporal feasibility of a project.
Polymorphism: Controlling the Crystalline Identity
The solid-state properties of an active pharmaceutical ingredient (API) can hold the key to a drug’s performance, and few aspects are as critical, or as challenging, as polymorphism.
Polymorphism refers to the ability of a solid compound to exist in two or more distinct crystalline forms, each having the same chemical composition but differing in their molecular arrangement within the crystal lattice . This phenomenon is remarkably common in active pharmaceutical ingredients, with over 50% of all APIs existing in at least two polymorphic forms .
Why does this matter? Different polymorphic forms can dramatically alter a drug’s physicochemical properties. These variations can impact critical attributes such as solubility, dissolution rate, melting point, density, flowability, hygroscopicity (tendency to absorb moisture), and compaction characteristics . Consequently, these changes can profoundly affect the drug’s bioavailability and overall stability. A stark illustration of this impact is the case of Norvir® (ritonavir), an antiretroviral drug. A transformation of its API into a less soluble polymorphic form led to its market removal, highlighting the serious public health and commercial consequences of uncontrolled polymorphism .
Given these critical implications, stringent control and characterization of polymorphic forms are paramount. The U.S. FDA, for instance, requires a comprehensive polymorphism study as part of Abbreviated New Drug Application (ANDA) submissions to demonstrate equivalence to the reference listed drug . A suite of advanced analytical techniques is employed for identifying, characterizing, and quantifying these polymorphic forms:
- X-ray Powder Diffraction (XRPD): Provides unequivocal proof of polymorphism by analyzing the unique diffraction pattern of each crystal form .
- Thermal Analysis Techniques: Including Differential Scanning Calorimetry (DSC), Thermal Gravimetric Analysis (TGA), and Hot-Stage Microscopy (HSM), which reveal thermal transitions (melting, decomposition) and changes in mass associated with different polymorphs .
- Spectroscopic Methods: Raman spectroscopy and solid-state carbon-13 nuclear magnetic resonance (NMR-13C) provide insights into molecular structure and bonding within the crystal lattice .
Beyond initial characterization, continuous monitoring of polymorphic forms is essential throughout the drug development and manufacturing lifecycle to ensure consistent quality and prevent unintended transformations .
Polymorphism, therefore, is not merely a scientific curiosity; it is an intellectual property battleground and a constant quality control minefield. Innovator companies often patent-protect different crystalline forms of their APIs, creating complex “patent thickets” that generic developers must meticulously navigate . An unintentional change in polymorphic form during manufacturing, storage, or even due to mechanical stress (e.g., during tablet compression), can compromise a product’s performance, lead to batch failures, or, in severe cases, necessitate product recalls, posing significant public health concerns . This elevates polymorphism from a formulation hurdle to a multi-faceted strategic and operational risk that demands continuous vigilance and advanced analytical capabilities.
Stability and Degradation: Ensuring Shelf-Life and Integrity
A drug’s therapeutic utility is inextricably linked to its stability—its ability to maintain its intended properties over time. For generic drugs, demonstrating robust stability is a non-negotiable regulatory and quality requirement.
Drug stability refers to the capacity of a drug dosage form to retain its chemical, physical, and microbiological integrity, potency, and purity throughout its shelf-life when stored under specified environmental conditions, such as temperature, humidity, and light . This property is absolutely crucial for ensuring that the product remains safe and effective for patients from the moment of manufacture until its expiration date .
Pharmaceutical drugs are susceptible to various degradation pathways, which can compromise their integrity and lead to the formation of impurities. The major degradation pathways include:
- Hydrolysis: Chemical breakdown caused by water, often accelerated by extreme pH conditions (acid or base hydrolysis) . This can lead to the loss of structural components necessary for the molecule’s activity.
- Oxidative Degradation: Reactions involving oxygen, often catalyzed by light or metal ions, leading to changes in chemical bonds or the formation of new compounds .
- Photodegradation: Chemical changes induced by exposure to light, which can cause molecules to break down and become ineffective or even toxic .
- Thermal Degradation: Exposure to elevated temperatures can cause molecules to lose critical functional groups or undergo other structural changes .
To counteract these degradation processes and ensure long-term stability, robust formulation and stability-indicating testing strategies are indispensable . Key stabilization strategies include:
- Strategic Excipient Selection: Incorporating excipients with stabilizing properties, such as antioxidants (e.g., ascorbic acid, tocopherol) to prevent oxidative degradation, and pH modifiers or buffering agents to maintain the desired pH and prevent hydrolysis of acid- or base-sensitive drugs .
- Advanced Formulation Techniques: Methods like microencapsulation, which encloses drug particles within a protective shell, and lyophilization (freeze-drying), which removes water from the formulation, can provide physical protection and minimize exposure to environmental factors that trigger degradation .
- Optimized Packaging: The choice of container-closure system is critical. Packaging must effectively protect the drug product from light, air (oxygen), and moisture throughout its shelf life .
The stability of a drug product is not an isolated property; it is deeply intertwined with the entire manufacturing process, the quality of raw materials (especially excipients), and the chosen packaging. Problems arising during manufacturing scale-up, such as uncontrolled process variability or the presence of impurities originating from excipients, can directly compromise product stability . For example, certain excipients may contain trace impurities (e.g., reducing sugars, peroxides, aldehydes) that can react with the API and promote degradation pathways like Maillard reactions or oxidation . This means that a holistic approach, from meticulous raw material sourcing and rigorous in-process controls to appropriate final packaging, is essential to ensure long-term product integrity and prevent costly batch failures or regulatory issues.
To summarize these core physicochemical challenges and their mitigation, consider the following:
| Challenge | Description of Impact | Key Mitigation Strategies |
| Solubility | Poor absorption, low bioavailability, limited therapeutic effect. | Particle size reduction (micronization, nano-milling), Amorphous Solid Dispersion (ASD), lipid-based formulations, salt formation, pH adjustment, surfactants. |
| Dissolution | Inconsistent drug release, bioequivalence failure, sub-therapeutic levels. | Formulation optimization (excipient selection), biorelevant dissolution testing, use of surfactants in media, design of experiments (DoE). |
| Polymorphism | Changes in solubility, dissolution, stability, bioavailability; IP infringement risk. | Analytical control (XRPD, DSC, Raman), selection of stable polymorphic form, continuous monitoring throughout manufacturing. |
| Stability/Degradation | Loss of potency/purity, formation of toxic impurities, reduced shelf-life, safety concerns. | Antioxidants, pH modifiers/buffers, microencapsulation, lyophilization, appropriate container-closure systems, stability-indicating analytical methods. |
The Excipient Enigma: Selection, Compatibility, and Impact
Often overlooked, the “inactive” components of a drug formulation—excipients—play an unexpectedly active role in determining a generic drug’s success or failure.
Navigating Excipient Functionality and Regulatory Acceptance
Excipients are far from inert fillers; they are crucial functional components of a drug formulation. They perform a multitude of roles, from providing bulk to make a tablet large enough to handle, preventing it from crumbling, and helping it dissolve in the stomach or intestine, to imparting a pleasant taste and color . Given their diverse functions, the selection of appropriate excipients is of paramount importance in generic formulations, directly influencing the risk profile and overall performance of the drug .
However, the “inactive” label can be misleading. Excipients can, and often do, interact with the active pharmaceutical ingredient (API), leading to undesirable chemical reactions or degradation . For instance, the presence of adsorbed water during storage, changes in microenvironmental pH within the dosage form, or the activation of functional groups (or even impurities) present in excipients can promote various drug degradation pathways . Specific examples include:
- Maillard Reactions: Excipients containing reducing sugars (e.g., lactose, maltose, glucose) or even trace amounts of reducing sugars as impurities (e.g., in microcrystalline cellulose) can promote Maillard reactions with primary and secondary amine-containing drugs .
- Oxidation/Photodegradation: Peroxides or phenolic impurities found in excipients like povidone and crospovidone can lead to the oxidation or photodegradation of the drug .
- Aldehyde-induced Degradation: Aldehydes present in excipients such as lactose, microcrystalline cellulose (MCC), starch, and polyethylene glycol (PEG) can also cause drug degradation .
Regulatory bodies, including the FDA, are keenly aware of these potential interactions. They require detailed information on the chemistry and characterization of inactive ingredients to ensure Q1 (qualitative) and Q2 (quantitative) sameness, especially for critical dosage forms like parenteral, ophthalmic, and otic products .
The choice and quality of excipients, even when seemingly Q1/Q2 compliant, can subtly influence a generic drug’s in vivo performance, often acting as hidden variables in the complex equation of bioequivalence. This can lead to unexpected bioequivalence failures or even unforeseen adverse effects in patients . The fact that excipients from different vendors or even different grades from the same vendor may contain varying impurities further compounds this challenge . This means generic developers must go beyond merely meeting the Q1/Q2 checkboxes; they must conduct rigorous excipient compatibility studies, as excipient-related issues can be a root cause of significant formulation challenges and regulatory setbacks.
Mitigating Drug-Excipient Interactions and Impurity Risks
Given the potential for excipients to compromise drug stability and performance, drug-excipient compatibility studies (DECS) are a critical step during pre-formulation studies. These studies aim to identify and select appropriate excipients that will yield a stable and effective formulation . Indeed, DECS are not just good scientific practice; they are an obligatory part of regulatory submissions for Abbreviated New Drug Applications (ANDAs) .
Traditionally, DECS methods have been labor-intensive and lengthy. They typically involve preparing binary or multi-component physical mixtures of drugs and excipients, which are then placed on stability under accelerated conditions (often with or without added water) for extended periods, usually one to three months . While thorough, this approach can be a significant bottleneck in the accelerated generic development cycle. This has spurred the search for more efficient methods. Novel approaches, such as the “vial-in-vial” method, have been proposed to rapidly screen suitable excipients, with the potential to shorten the drug development cycle by many folds .
Ensuring excipient compatibility with active pharmaceutical ingredients (APIs) in complex formulations presents a particularly significant challenge . For example, polysorbates, commonly used excipients in many complex formulations (especially biologics), are known to be prone to degradation. This degradation can occur through various pathways, including oxidation (e.g., due to peroxides or exposure to light and oxygen) and enzymatic degradation (e.g., by lipases) . Such degradation can lead to the formation of subvisible and visible particles, directly impacting the drug product’s critical quality attributes (CQAs) and potentially raising patient safety concerns .
The complexity and time-consuming nature of traditional DECS highlight a growing imperative for predictive excipient science. There is a clear need for advanced analytical techniques and computational models to anticipate drug-excipient interactions and identify impurity risks much earlier in the development process. This proactive approach, rather than relying on lengthy empirical testing, can significantly reduce costly late-stage failures, accelerate time-to-market, and ultimately enhance the overall efficiency and success rate of generic drug development programs.
Manufacturing and Scale-Up: Bridging Lab to Commercial Reality
The journey of a generic drug from a laboratory bench to mass production is a formidable undertaking, riddled with challenges related to process consistency and supply chain resilience.
Process Variability: Maintaining Consistency at Scale
For a generic drug to be approved and marketed, manufacturers must demonstrate that their chosen manufacturing process can reliably and consistently produce a high-quality product that is bioequivalent to the Reference Listed Drug (RLD) . This requires submitting detailed information in the Abbreviated New Drug Application (ANDA) regarding the drug’s formulation, composition, the specific manufacturing process, and comprehensive quality control measures .
However, the transition from small-scale laboratory formulation to commercial-scale manufacturing is often fraught with difficulties. This phase is frequently referred to as the “valley of death” in drug development due to the high rate of failure. For generic drugs, this challenge is exacerbated by the need to precisely match the RLD’s in vivo performance, often while utilizing different equipment, processes, or even raw material suppliers than the innovator . Common problems encountered during scale-up include wasted commercial batches, failure to meet established specifications, and unacceptable process variability .
To bridge this gap and ensure consistency, it is crucial to establish a clear and robust relationship between formulation variables, manufacturing process parameters (including attributes of the drug substance and excipients), and the final product quality . This involves meticulously identifying the sources of variability at each step of the manufacturing process and implementing controls to mitigate them. Without this deep process understanding, achieving reproducible quality and bioequivalence at commercial scale remains a significant hurdle.
Supply Chain Vulnerabilities: Ensuring Raw Material Flow
The globalized nature of the pharmaceutical industry, while offering cost advantages, has also introduced significant vulnerabilities into the supply chain, directly impacting generic drug formulation and production.
Generic drug manufacturers frequently encounter substantial supply chain constraints, including shortages of critical ingredients and limitations in manufacturing capacity, both of which can severely impede the timely delivery of products to market . A major contributing factor to this fragility is the industry’s widespread reliance on offshore manufacturing for active pharmaceutical ingredients (APIs) and other essential components. Startlingly, over 80% of active pharmaceutical ingredients used in the U.S. are manufactured outside the country, with a significant portion originating from China and India .
This globalized supply chain, while initially driven by cost reduction (lower labor costs, less stringent regulatory standards in some regions), comes at a considerable price . It exposes generic manufacturers to a range of geopolitical risks, including trade policies like tariffs, and potential disruptions stemming from regional crises or quality control issues in foreign facilities .
The implication here is profound: geopolitical risks can directly translate into acute formulation challenges for generic manufacturers. If tariffs are imposed on critical imported ingredients, or if a regional crisis disrupts the supply of a specific excipient or API, generic companies face a difficult choice. They must either reformulate their product (a complex and time-consuming process that requires re-demonstrating bioequivalence and obtaining regulatory approval, if even permissible) or risk drug shortages. This directly impacts patient access and can severely erode a company’s profitability. This elevates supply chain management from a logistical concern to a strategic imperative that directly influences formulation decisions and overall business resilience.
Specialized Formulation Challenges Across Dosage Forms
While the fundamental principles of generic drug development apply universally, specific dosage forms present unique and often complex formulation challenges that demand specialized expertise and innovative solutions.
Modified-Release (MR) Formulations: Precision in Drug Delivery
Modified-release (MR) formulations represent a significant leap in drug delivery, offering enhanced patient convenience and improved therapeutic outcomes. However, developing generic versions of these complex systems is a formidable task.
Sustained, Delayed, and Pulsatile Release: Complexities of Control
MR products are sophisticated dosage forms meticulously engineered to control the rate, time, or location of drug release within the body. Their primary objective is to achieve desired efficacy and safety profiles, often by maintaining stable drug concentrations in the bloodstream over extended periods, thereby avoiding the undesirable “peaks and troughs” associated with immediate-release formulations . This controlled release can significantly improve patient compliance by reducing the frequency of dosing . Examples include prolonged-action, sustained-release, and delayed-release formulations, each designed to meet specific therapeutic needs . These systems frequently rely on specialized polymers and intricate designs to orchestrate the precise release profile .
However, the very precision that defines MR formulations also introduces their greatest challenge. Inappropriate control of drug release can lead to severe consequences, ranging from reduced therapeutic efficacy (if the drug is released too slowly or incompletely) to increased toxicity (if the drug is released too rapidly) . This is often referred to as the “Goldilocks” challenge of MR formulations: the release profile must be just right—not too fast, not too slow, but precisely controlled to remain within the narrow therapeutic window. Achieving this delicate balance requires a profound understanding of drug-polymer interactions, the physicochemical properties of the API, and the complex physiology of the gastrointestinal (GI) tract. It’s a scientific tightrope walk that demands meticulous design and rigorous testing.
Overcoming Dose Dumping and Food Effects
Beyond the inherent complexities of controlled release, MR generic formulations must contend with dynamic physiological variables, notably the risks of “dose dumping” and “food effects.”
Dose dumping is a critical safety concern for MR formulations. It occurs when a large, potentially toxic, amount of medication is released rapidly and prematurely from the dosage form, leading to dangerously high drug concentrations in the bloodstream . This can happen if the formulation’s integrity is compromised (e.g., by alcohol interaction, or mechanical stress) or due to specific physiological conditions. The consequences can range from severe adverse effects to outright toxicity. For example, some generic versions of extended-release Concerta, used to treat ADHD, were found not to dissolve at the same rate as the brand, leading to inconsistent doses and potentially dose dumping .
Food effects represent another significant challenge. The presence of food in the gastrointestinal tract can profoundly impact the absorption of MR formulations, potentially altering gastric emptying rates, GI transit times, and local pH conditions, all of which can disrupt the intended drug release profile . For instance, a high caloric meal can significantly delay gastric emptying, retaining a drug in the stomach for an extended period. If an MR formulation is designed to release its active ingredient in the small intestine, prolonged gastric retention due to food could lead to an unintended and premature release in the stomach, altering the PK profile and potentially causing adverse events . To mitigate these risks, food effect studies are strongly recommended early in the development of new MR formulations to inform appropriate intake recommendations (e.g., take with or without food) that can be included in the product labeling .
The variability inherent in human physiology, particularly the dynamic environment of the gastrointestinal tract (e.g., fed versus fasted states, individual differences in motility and pH), presents a significant and often unpredictable challenge for MR generic formulation. Designing a robust MR product means engineering it to perform consistently despite these inherent physiological fluctuations. This demands not only sophisticated formulation design but also extensive and often costly in vivo studies to characterize drug release under various conditions and ensure that the product is resilient to physiological variability . It is a complex endeavor that pushes the boundaries of pharmaceutical science to ensure consistent therapeutic outcomes for patients.
Parenteral Formulations: The Demands of Injectables
Parenteral drug products, administered directly into the body, offer rapid and precise drug delivery but impose exceptionally stringent formulation and manufacturing requirements on generic developers.
Sterility, Pyrogenicity, and Isotonicity: Non-Negotiable Standards
Parenteral drugs, which include intravenous, intramuscular, and subcutaneous injections, bypass the body’s natural defense mechanisms, delivering medication directly into the systemic circulation or tissues . This direct route of administration necessitates exceptionally stringent regulatory requirements, far exceeding those for oral medications .
Three non-negotiable standards define the landscape of parenteral formulation:
- Sterility: Parenteral formulations must be absolutely free of microorganisms and contaminants . Achieving this requires manufacturing under strictly sterile conditions, often within highly controlled clean rooms utilizing advanced aseptic manufacturing systems . This demands exhaustive investments in specialized equipment and facilities, as existing units used for other formulations often cannot be directly repurposed .
- Pyrogenicity: Beyond sterility, parenteral products must be pyrogen-free, meaning they are devoid of fever-inducing substances, primarily bacterial endotoxins . Ensuring this requires meticulous control throughout the manufacturing process, often employing advanced systems like microfluidic biosensors and affinity chromatography for real-time endotoxin detection and removal .
- Isotonicity: The final parenteral formulation must possess the same osmolarity (concentration of solutes) as human body fluids to prevent cell damage, pain, or irritation at the injection site . This delicate balance is typically adjusted using osmolality-modifying agents such as sodium chloride or dextrose .
The non-negotiable demands for absolute purity, pyrogen-free status, and physiological compatibility in parenteral formulations translate directly into significantly higher manufacturing costs and infrastructure requirements for generic developers compared to oral solids. This creates a substantial barrier to entry, explaining why there are often fewer generic competitors in the injectable segment . This is the cost of absolute purity—a necessary investment to ensure patient safety when the body’s natural defenses are bypassed.
Stability of Biologics and Complex Injectables
Beyond the fundamental requirements of sterility and pyrogenicity, maintaining the stability of parenteral formulations, particularly complex injectables and biologics, presents its own set of formidable challenges.
Parenteral drugs, especially biologics (large, complex protein-based molecules), are inherently prone to degradation . Their complex structures are highly sensitive to environmental factors such as temperature, pH, humidity, and light. Maintaining their stability requires meticulous control of these conditions not only throughout the manufacturing process but also during transport (often necessitating expensive cold-chain logistics) and storage .
The pharmaceutical industry has witnessed rapid growth in the area of complex injectable products, including novel formulations like nanoparticles, liposomes, microspheres, suspensions, and emulsions . These advanced delivery systems are increasingly developed for parenteral applications due to their ability to offer improved pharmacokinetic/pharmacodynamic (PK/PD) behavior, reduced dosing frequency, and minimal adverse effects . For example, long-acting injectables (LAIs) can provide sustained drug release for prolonged durations, overcoming limitations of conventional formulations such as low adherence and frequent dosing requirements .
For parenteral generics, particularly complex injectables, formulation choices extend beyond mere drug stability and bioavailability; they directly influence patient experience and, ultimately, adherence. This means successful formulation goes beyond achieving bioequivalence to encompass a holistic, patient-centric design. Consider the shift towards pre-filled syringes and auto-injectors. While their regulatory approval can be more complex, these specialized delivery systems offer significant benefits at the point of use, including fewer administration errors, reduced likelihood of contamination, and easier self-administration by patients or their caregivers . This demonstrates a crucial interplay of formulation, delivery, and patient adherence, where the formulation is designed not just for efficacy but also for optimal patient use, becoming a key differentiator in the market.
Topical and Ophthalmic Formulations: Local Action, Systemic Hurdles
Topical and ophthalmic formulations are designed for localized drug delivery, yet they face unique challenges in permeating biological barriers while minimizing systemic exposure.
Skin and Ocular Barriers: Enhancing Permeation and Retention
Topical formulations, applied to the skin, and ophthalmic formulations, applied to the eye, are intended to exert their therapeutic effects locally. However, achieving effective drug delivery to the target tissue is profoundly challenging due to the formidable natural barriers posed by the skin and the intricate physiology of the eye .
For ophthalmic drugs, low ocular bioavailability is a major limitation. The eye’s unique anatomy and physiology present multiple barriers:
- Limited Volume: The eye can only accommodate a small volume (around 30 µL) of eye drop, with most of the excess volume rapidly eliminated by reflex blinking .
- Tear Film Turnover: The rapid turnover of the tear film (0.5 to 2.2 µL/min) quickly clears drug molecules via nasolacrimal drainage .
- Permeation Barriers: Tight junctions between epithelial cells form a strong barrier for hydrophilic drugs, while drug efflux pumps and drug-degrading enzymes (e.g., cytochrome P450) in the epithelium further limit bioavailability .
To overcome these barriers and enhance drug permeation and retention, generic developers employ innovative formulation strategies:
- Viscosity Modifiers: Incorporating polymers such as hydroxypropyl methylcellulose (HPMC), hydroxyethyl cellulose (HEC), gellan gum, or xanthan gum can increase the viscosity of ophthalmic formulations. This prolongs contact time with the ocular tissue and reduces rapid drainage, thereby enhancing drug efficacy .
- Permeation Enhancers: The addition of surfactants, bile acids, or chelating agents can increase corneal penetration . Prodrug formulations, which are pharmacologically inactive but more lipophilic derivatives of the drug, can also enhance penetration by metabolizing to the active compound within the cornea after absorption .
- Nanotechnology: Nanosuspensions, for instance, can improve the bioavailability of poorly soluble (hydrophobic) drugs by increasing their solubility and extending their residence time on the ocular surface .
This leads to a fascinating paradox: for topical and ophthalmic generics, the challenge isn’t simply delivering the drug; it’s ensuring sufficient local bioavailability to achieve the therapeutic effect without significant systemic absorption. This delicate balance requires innovative formulation strategies that prioritize local efficacy while minimizing systemic side effects. Simple bioequivalence metrics, often sufficient for systemic drugs, are frequently inadequate for these localized delivery systems, necessitating sophisticated in vitro and in vivo studies, such as cutaneous pharmacokinetics, to precisely characterize drug action at the target site .
Formulation Viscosity and Particle Size: Impact on Efficacy
For topical and ophthalmic formulations, seemingly minor micro-level attributes, such as the particle size of the active pharmaceutical ingredient (API) and the viscosity of the excipient matrix, can have a profound impact on the drug product’s macro-level performance and therapeutic efficacy.
In topical formulations like creams or ointments, the particle size or morphology of the drug substance and the consistency (viscosity) of the white petrolatum or other excipient bases are considered critical quality attributes . Any deviation in these parameters can lead to a shift in content uniformity (ensuring even drug distribution), drug release, and the desired dermal distribution of the drug .
Similarly, in ophthalmic suspensions, the particle size of the API directly influences its residence time and activity on the precorneal surface . Smaller particles may replenish the drug absorbed by ocular tissue more quickly, while larger particles might be retained longer, slowing drug dissolution. However, excessively large particles can also cause practical problems, such as clogging the tip of the medication bottle during instillation, which directly affects dosage consistency and the delivered drug concentration . Studies have shown differences in particle sizes between generic and brand-name ophthalmic products, potentially impacting efficacy .
This highlights a crucial principle: for these localized dosage forms, the micro-level determinants of macro-level performance are paramount. Achieving therapeutic equivalence requires extremely precise control over material attributes and manufacturing processes. Generic developers must meticulously characterize not only the chemical composition but also the physical microstructure and rheological properties (e.g., flow behavior, viscosity) of the Reference Listed Drug (RLD) to ensure their generic product delivers the same consistent and effective dose to the target site.
Inhalation Formulations: The Device-Drug Interplay
Inhalation drug products (IDPs) offer a direct route to the lungs for treating respiratory conditions or achieving systemic effects. However, developing generic IDPs is exceptionally complex, primarily due to the inextricable link between the drug formulation and its delivery device.
Aerosol Properties and Particle Size Distribution: Delivering to the Lungs
Pulmonary drug delivery is inherently a “combination product”—it involves integrating a drug formulation with a specialized delivery device, such as pressurized metered-dose inhalers (pMDIs), dry powder inhalers (DPIs), or nebulizers . The primary objective of an inhalation product is to efficiently deliver the active pharmaceutical ingredient (API) to the patient’s nose, airways, or lungs .
Achieving this precise delivery requires meticulous control over the drug product’s aerosol properties and particle size distribution. The size and characteristics of the aerosolized particles directly determine where the drug will deposit within the respiratory tract (e.g., upper airways, large bronchi, or deep lung alveoli), which is critical for its therapeutic effect .
To gain regulatory approval, generic inhalation drug products must demonstrate pharmaceutical equivalence (same active ingredients, dosage form, strength, route of administration), bioequivalence (no significant difference in drug absorption and action), and therapeutic equivalence (same clinical effect and safety profile) as the Reference Listed Drug (RLD) . The FDA employs a comprehensive “weight-of-evidence” model for this assessment, which includes:
- In vitro studies: Comparing aerosol properties, particle size distribution, and delivered dose of the generic and RLD .
- Pharmacokinetic (PK) studies: Evaluating systemic exposure .
- Pharmacodynamic (PD) or clinical endpoint studies: Assessing the therapeutic effect, especially when PK studies alone are insufficient .
The delivery device is not merely a container for inhalation generics; it is an integral component of the formulation that critically influences drug performance. Variations in device design, manufacturing tolerances, or even the materials used can directly alter the aerosol properties and particle size distribution, leading to different drug delivery profiles and potential bioequivalence failures . This means generic developers cannot simply copy the drug; they must also replicate or design a device that ensures equivalent drug delivery performance, effectively making the device a “critical excipient” in its own right.
Device-Specific Challenges: pMDIs, DPIs, and Nebulizers
Developing inhalation products is notoriously costly and time-consuming, carrying a significant risk of major delays and increased development costs . This complexity stems from several factors unique to these drug-device combinations:
- Conflicting User Requirements: The device must balance diverse and often conflicting demands from users, payers, and healthcare professionals. For example, it needs to be safe against inadvertent actuation when carried in a pocket, yet easy to open and operate by a patient with impaired vision or dexterity .
- Product Complexity: The chemical and physical properties of the drug, along with its pharmacodynamic and pharmacokinetic characteristics, profoundly influence the selection of the most suitable formulation and device type. The drug’s potency dictates dose size, and the formulation must be chemically and physically stable within the device for an extended shelf-life, often requiring protection from moisture, light, and oxygen .
- Inextricable Performance: The performance of an inhalation product is an inseparable function of both the drug formulation and the device. Any change in one component can impact the other, making optimization a complex iterative process.
The complexity of inhalation devices, coupled with the lack of direct substitutability concepts in some regulatory regions (e.g., the EU, where approval depends on demonstrating drug delivery equivalence rather than direct interchangeability), means generic developers face a regulatory “black box” . They must not only prove bioequivalence but also demonstrate equivalent drug delivery performance through a battery of in vitro, PK, and potentially PD or clinical studies. This is often done without clear, harmonized global guidelines for device-specific parameters, making the regulatory pathway less predictable and significantly more resource-intensive. It’s a challenging landscape where scientific rigor meets regulatory ambiguity, demanding a high level of expertise and investment.
Biosimilars: The Pinnacle of Complex Generic Development
Biosimilars represent the most advanced and challenging frontier in generic drug development, pushing the boundaries of analytical science and regulatory oversight.
Demonstrating High Similarity: Beyond Bioequivalence
Biosimilars are generic versions of complex biologic drugs, which are typically large, intricate molecules derived from living organisms (e.g., proteins, monoclonal antibodies) . Unlike small-molecule generics, which must contain the “same chemical substance” as their reference product, biosimilars are fundamentally not identical to their reference biologics . This crucial distinction arises from the inherent variability of recombinant proteins and the subtle differences that inevitably occur during their complex production processes. Factors like the specific expression system used (e.g., Chinese Hamster Ovary (CHO) cells vs. Human Embryonic Kidney 293 (HEK293) cells), cell culture conditions (pH, oxygen, nutrients), and bioprocessing variables (purification methods, buffer composition) can all lead to structural nuances .
Therefore, biosimilars are not required to demonstrate strict bioequivalence in the same way as small-molecule generics. Instead, they must demonstrate “high similarity” to the reference biologic. This requires a comprehensive “totality of evidence” approach, which is a far more extensive and demanding process. This evidence package includes:
- Extensive Analytical and Physicochemical Characterization: High-resolution techniques are used to compare the primary protein sequence, three-dimensional structure, and other physical attributes .
- Biologic and Functional Characterization: Studies to compare biological activity, binding affinity, and mechanism of action .
- Non-clinical Studies: Including comparative toxicity assessments in animal models .
- Clinical Trials: Often required to confirm comparability in terms of safety, efficacy, and immunogenicity in human subjects, especially if any residual uncertainty remains after analytical and non-clinical studies .
Developing biosimilars is akin to replicating a complex biological fingerprint, not just a simple chemical formula. The profound challenge lies in ensuring that these slight variations in complex structures—such as post-translational modifications (PTMs) like glycosylation patterns—do not lead to clinically significant differences in efficacy, safety, or, critically, immunogenicity. Even minor differences in these complex biological attributes can trigger an unwanted immune response in patients, making the “fingerprint” challenge a high-stakes endeavor for biosimilar developers .
Post-Translational Modifications and Immunogenicity Concerns
The most formidable and often unpredictable challenge in biosimilar development is managing post-translational modifications (PTMs) and addressing potential immunogenicity.
Achieving consistent post-translational modification (PTM) profiles, such as glycosylation patterns (the attachment of sugar molecules to proteins), is a major hurdle for biosimilars. These modifications are highly sensitive to the specific cellular machinery and environmental conditions during protein production. Differences in expression systems, cell culture conditions (e.g., pH, oxygen, nutrient availability), and bioprocessing variables (e.g., purification methods, buffer composition) can all lead to variations in PTMs between the biosimilar and its reference product .
These subtle structural nuances necessitate strict regulatory oversight to ensure that the biosimilar is truly therapeutically equivalent to its reference counterpart in terms of efficacy, safety, and quality . However, the most significant concern arising from these variations is immunogenicity—the potential for the human body to develop an immune response against the biosimilar drug. This immune response can range from benign (e.g., development of anti-drug antibodies that do not affect efficacy) to severe (e.g., neutralization of the drug’s therapeutic effect, leading to loss of efficacy, or serious adverse events like anaphylaxis or autoimmune reactions) .
The unpredictable nature of the immune response poses a significant safety risk and is a primary driver of the extensive and costly clinical studies required for biosimilar approval . Even if analytical and functional studies show high similarity, a clinical trial is often required to rule out clinically meaningful differences in immunogenicity. This makes biosimilar development a high-risk, high-reward endeavor. The challenge is not just to produce a similar molecule, but to ensure that the body perceives it as therapeutically interchangeable, without triggering an unwanted immune reaction.
Pioneering Solutions and Innovative Strategies
The formidable challenges in generic drug formulation are met with equally powerful solutions, driven by a proactive approach to quality, advanced analytical capabilities, computational prowess, and novel drug delivery systems.
Quality by Design (QbD) and Process Analytical Technology (PAT): A Proactive Paradigm
Traditional pharmaceutical development often relied on empirical approaches and extensive end-product testing to ensure quality. However, the increasing complexity of drug products and the demand for efficiency have spurred the adoption of a more proactive paradigm: Quality by Design (QbD) and Process Analytical Technology (PAT).
Implementing QbD for Robust Formulation Development
Quality by Design (QbD) is a systematic approach to pharmaceutical development that fundamentally shifts the focus from “testing quality in” to “designing quality in” from the very beginning of the development process . It emphasizes a deep understanding of the product and the manufacturing process, all based on sound scientific principles and robust quality risk management .
The implementation of QbD involves several key elements:
- Quality Target Product Profile (QTPP): This is a prospective summary of the desired quality characteristics of the drug product, defined with patient safety and efficacy in mind . It sets the ultimate goal for the formulation.
- Critical Quality Attributes (CQAs): These are measurable properties of the drug product that are critical to ensuring its quality, safety, purity, and efficacy from the patient’s perspective. CQAs are directly derived from the QTPP .
- Critical Material Attributes (CMAs) and Critical Process Parameters (CPPs): CMAs are the physical, chemical, biological, or microbiological properties of input materials (e.g., APIs, excipients) that must be controlled to ensure the desired quality of the output material. CPPs are the process parameters (e.g., mixing speed, temperature, pressure) whose variability has an impact on a CQA and therefore should be monitored or controlled to ensure the process produces the desired quality .
- Risk Assessment and Design of Experiments (DoE): QbD employs tools like Quality Risk Management (e.g., Failure Mode and Effects Analysis – FMEA) to prospectively identify potential risks to product quality. This is followed by Design of Experiments (DoE), a statistical methodology used to systematically study the effects of multiple variables (CMAs and CPPs) on product and process performance, enabling optimization and robust development .
For generic drugs, QbD is particularly transformative. It moves development from an empirical, trial-and-error process, which can be labor-consuming, tedious, and costly , to a knowledge-driven, proactive one. By systematically understanding the relationships between formulation components, manufacturing process variables, and product quality, companies can identify the precise variables that need adjustment to achieve bioequivalence and consistently reduce product variability . This approach significantly reduces development timelines, minimizes costly failures (e.g., failed bioequivalence studies or batch rejections), and enhances product robustness, thereby mitigating regulatory risks and improving overall cost-efficiency . QbD is not just a scientific methodology; it is a strategic business tool for de-risking and accelerating development by shifting from a reactive “test-and-fix” mindset to a proactive “design-for-quality” paradigm.
PAT: Real-Time Monitoring for Enhanced Quality Control
Complementing Quality by Design (QbD) is Process Analytical Technology (PAT), a framework for designing, analyzing, and controlling pharmaceutical manufacturing processes through timely measurements of critical quality and performance attributes of raw and in-process materials and processes.
PAT involves the real-time monitoring and control of manufacturing processes, ensuring consistent product quality and significantly reducing product variability . It integrates seamlessly with QbD principles by providing the tools necessary for enhanced process understanding and control. PAT tools encompass a range of advanced analytical techniques that can provide real-time data on critical process variables. Examples include Raman spectroscopy, Near-Infrared (NIR) spectroscopy, and Fourier-Transform Infrared (FTIR) spectroscopy, which can monitor parameters like pH, osmolality, and API concentration directly within the manufacturing line .
By implementing PAT, generic manufacturers can achieve a new level of quality assurance. This technology allows for immediate detection of deviations, enabling prompt corrective actions, which in turn enhances product consistency, reduces batch failures, and minimizes production variability . These quality improvements not only mitigate regulatory risks but also serve as powerful competitive differentiators in markets where quality concerns have historically impacted confidence in certain generic products.
PAT represents a crucial step towards the digital transformation of generic drug manufacturing. It signifies a shift from discrete batch testing, where quality is assessed after production, to continuous, real-time quality assurance, where quality is built into and monitored throughout the process. This paradigm shift enables more agile and efficient manufacturing operations, which is vital in a highly competitive, cost-sensitive market. Moreover, it lays the groundwork for future advancements like continuous manufacturing, fundamentally changing how drugs are produced and how their quality is ensured.
Advanced Analytical Characterization: Deeper Insights into Drug Products
In the pursuit of bioequivalence and therapeutic equivalence, generic drug developers rely heavily on sophisticated analytical techniques to gain profound insights into both the Reference Listed Drug (RLD) and their own proposed generic product.
High-Resolution Techniques for Structural and Physicochemical Analysis
Analytical techniques are the backbone of drug development, holding a critical position in bioequivalence studies by enabling the precise measurement of a drug’s active pharmaceutical ingredient (API) presence and concentration within the human body . However, as innovator drug products become increasingly complex—especially biologics and nano-sized products with intricate supra-molecular structures like protein-drug complexes or iron colloids—the demands on analytical sophistication have escalated . Merely replicating a chemical formula is no longer sufficient; generic developers must employ cutting-edge high-resolution techniques to characterize subtle structural and physicochemical differences that could profoundly impact drug performance.
Comprehensive physicochemical characterization is required to gain deep insight into product quality that can eventually affect in vivo performance . This includes meticulous assessment of properties such as particle morphology, surface potential, size distribution, carrier composition, and the precise physical state of the encapsulated drug .
A suite of advanced analytical techniques is indispensable for this level of detail:
- X-ray Powder Diffraction (XRPD): Used to identify and quantify crystalline forms (polymorphs) and assess overall crystallinity .
- Thermal Analysis (Differential Scanning Calorimetry – DSC, Thermal Gravimetric Analysis – TGA, Hot-Stage Microscopy – HSM): Provides information on thermal transitions, phase changes, and stability, crucial for understanding polymorphic behavior and degradation .
- Spectroscopic Methods (Raman spectroscopy, Solid-state Carbon-13 Nuclear Magnetic Resonance – NMR-13C): Offer detailed insights into molecular structure, bonding, and arrangement within the solid state .
- Particle Size Analyzers: Essential for characterizing the size distribution of drug particles, which is critical for dissolution and absorption, especially for poorly soluble drugs .
This increasing complexity of RLDs is driving an “arms race” in analytical sophistication for generic developers. The challenge is not just to possess these advanced tools, but to have the expertise to use them to detect subtle, performance-critical differences that are not immediately obvious. This pushes the boundaries of analytical science and necessitates significant investment in analytical R&D capabilities.
Bioanalytical Methods for Bioequivalence Assessment
The ultimate proof of a generic drug’s therapeutic equivalence often lies in its bioequivalence (BE) profile in human subjects. This requires robust bioanalytical methods to accurately quantify drug concentrations in biological samples over time.
Bioequivalence studies typically involve in vivo studies in human subjects where the pharmacokinetic (PK) profiles of the generic (test) drug and the reference drug are characterized and compared . The key PK parameters assessed are the peak drug plasma concentration (Cmax), which is a measure of the rate of absorption, and the area under the drug plasma concentration versus time profile (AUC), which reflects the extent of absorption .
To accurately measure the presence and concentration of the active pharmaceutical ingredient (API) and its metabolites in biological samples (e.g., blood plasma), a range of highly sensitive and specific bioanalytical techniques are employed:
- Liquid Chromatography-Mass Spectrometry (LC-MS): Widely regarded for its precision, high sensitivity, and specificity, LC-MS is adept at separating and quantifying drugs and their metabolites in complex biological matrices .
- Gas Chromatography-Mass Spectrometry (GC-MS): A valuable tool, particularly for drugs that are volatile or can be derivatized to a gaseous state, making it suitable for compounds with high volatility and low molecular weight .
- High-Performance Liquid Chromatography (HPLC): A versatile and foundational analytical method, HPLC is a workhorse for precisely separating and quantifying APIs. Reverse-phase HPLC is well-suited for hydrophobic drugs, while normal-phase HPLC is preferred for polar compounds .
- Immunoassays (e.g., Enzyme-Linked Immunosorbent Assay – ELISA): Crucial for analyzing larger molecules such as proteins and peptides, these methods rely on the specific binding of antibodies to drug molecules, making them ideal for high-throughput quantification of biologics and biosimilars .
- Capillary Electrophoresis and UV-Visible Spectrophotometry: Other methods employed, recognized for high resolution, swift analysis times (Capillary Electrophoresis), and measuring light absorption properties (UV-Vis) .
The sheer volume and complexity of bioanalytical data generated from these rigorous BE studies necessitate advanced data analytics and statistical modeling. This shift moves beyond simple pass/fail criteria to a more nuanced, predictive understanding of drug performance. It paves the way for virtual bioequivalence (VBE) simulations and reduces the need for extensive human testing, optimizing resource allocation and accelerating development timelines. This highlights the growing importance of computational and statistical expertise in generic drug development, transforming raw data into actionable intelligence.
Computational Modeling and In Silico Prediction: Accelerating Development
The pharmaceutical industry is increasingly turning to computational modeling and in silico (computer-based) prediction tools to streamline drug development, reduce costs, and accelerate market entry for generic drugs. These technologies offer a powerful alternative to traditional, often time-consuming and expensive, experimental approaches.
Physiologically Based Pharmacokinetic (PBPK) Modeling: Virtual Bioequivalence
Physiologically Based Pharmacokinetic (PBPK) modeling represents a sophisticated scientific approach that establishes predictive relationships between laboratory-based drug release profiles (in vitro) and pharmacokinetic behavior in humans (in vivo) . These mathematical models function as knowledge management systems, integrating scientific understanding and existing data across various aspects of drug products, including formulation, in vitro and in vivo release, pharmacokinetics (PK), pharmacodynamics (PD), and even clinical responses .
At its core, PBPK modeling simulates how a chemical is absorbed, distributed, metabolized, and excreted (ADME) over time within the human body. It achieves this by incorporating detailed physiological and biochemical characteristics, such as organ size, blood flow rates, and metabolic enzyme activities, alongside drug-specific properties like solubility, permeability, and protein binding .
The applications of PBPK modeling in generic drug development are transformative:
- Bioequivalence (BE) Assessment: PBPK modeling can support relative bioavailability (BA) and BE assessments, particularly valuable in challenging populations like pediatrics where extensive clinical studies are often not feasible or ethical . It can provide confidence in extrapolating adult BE results to pediatric populations, for example .
- Predicting Impact of Changes: It can effectively predict the in vivo effects of minor formulation changes, manufacturing process alterations, or even changes in manufacturing facilities . This predictive capability can significantly reduce the need for additional, costly in vivo studies, potentially supporting biowaivers .
- Regulatory Acceptance: The FDA has demonstrated a willingness to approve generic drugs using PBPK modeling to support alternative BE approaches. A notable success story is the approval of generic diclofenac sodium topical gel, 1%, where PBPK modeling and simulation supported an alternative BE approach in lieu of a comparative clinical endpoint BE study, leading to first-cycle approval .
PBPK modeling serves as a crucial bridge, connecting in vitro data to in vivo outcomes and enabling a “virtual” testing environment. This capability fundamentally reshapes the generic drug development paradigm, moving it towards a more efficient, science-driven, and ethical approach by significantly reducing the reliance on traditional, expensive, and time-consuming human clinical trials . It is a cornerstone of “model-informed drug development” (MIDD), aiming to decrease development uncertainty, cost, time, attrition, and failure rates .
In Silico Tools for Formulation Optimization and ADME Prediction
Beyond PBPK modeling, a broader suite of in silico (computer simulation) tools is revolutionizing generic drug development, offering unprecedented capabilities for formulation optimization and drug property prediction.
In silico modeling and simulation solutions are proving immensely valuable for Abbreviated New Drug Application (ANDA) programs. These computational methods can effectively predict the behavior of a drug within the human body, identify potential safety concerns early in development, and optimize dosing regimens, thereby streamlining the overall drug development process and reducing the need for costly and time-consuming clinical trials .
Specific applications of these advanced computational tools include:
- Formulation Optimization: By simulating how different formulation components interact and influence drug behavior in the body, these models can guide the development of effective and safe drug products, reducing the number of physical experiments required .
- ADME Predictions: In silico tools can accurately predict a compound’s absorption, distribution, metabolism, and elimination (ADME) properties. This provides crucial insights into potential safety issues, helps optimize pharmacokinetic profiles, and significantly reduces the time and cost associated with traditional ADME studies .
- Dissolution Safe Space: Computational models can assess the rate and extent to which a drug dissolves in a specific medium in silico, helping to define a “safe space” for dissolution parameters that ensures consistent performance .
- Inter-species PBPK Modeling: These models can predict how a drug or chemical will be distributed, metabolized, and eliminated across different species, including humans, thereby reducing the need for extensive animal testing and accelerating early-stage development .
The integration of Artificial Intelligence (AI) and Machine Learning (ML) with in silico modeling is poised to further revolutionize generic drug formulation. These cutting-edge technologies can analyze vast and complex datasets, uncover subtle patterns that might escape human detection, and dramatically improve prediction accuracy . This accelerates formulation optimization by rapidly screening potential candidates and reducing experimental iterations, offering a significant competitive edge in a fast-paced market. It represents a shift from merely predictive modeling to prescriptive design, where AI/ML can suggest optimal formulations based on desired performance characteristics.
Novel Drug Delivery Systems: Expanding Therapeutic Horizons
As the pharmaceutical landscape evolves, generic developers are increasingly leveraging novel drug delivery systems to overcome formulation challenges, enhance therapeutic profiles, and differentiate their products beyond mere price competition.
Nanotechnology: Enhancing Solubility, Bioavailability, and Targeting
Nanotechnology, involving the engineering of materials at the nanoscale (typically between 1 and 100 nanometers), has emerged as a groundbreaking solution for improving drug delivery, efficacy, and safety . This revolutionary technology is rapidly transforming how pharmaceutical products are developed and delivered, offering new possibilities for managing chronic conditions and addressing unmet medical needs.
The benefits of nanotechnology for generic drug development are multifaceted and significant:
- Enhanced Solubility and Bioavailability: Many active pharmaceutical ingredients (APIs) suffer from poor water solubility, which severely limits their absorption in the body. Nanoparticles can encapsulate these poorly soluble APIs, protecting them from degradation and significantly enhancing their solubility and bioavailability . Lipid-based nanocarriers, such as micelles and liposomes, are particularly effective at overcoming the acidic environment of the gastrointestinal tract, thereby enabling the oral administration of drugs that were previously limited to injectable routes .
- Targeting and Precision: One of nanotechnology’s most compelling advantages is its ability to direct drugs to specific tissues or cells. By attaching targeting ligands to nanoparticles, scientists can deliver drugs directly to diseased areas, minimizing off-target effects and significantly reducing systemic side effects . This precision is especially crucial in fields like oncology, where minimizing damage to healthy tissues is paramount.
- Controlled Release: Nanoparticle-based systems can be engineered to enable sustained drug release over extended periods, reducing the need for frequent dosing and improving patient adherence .
The market for nanotechnology drug delivery is projected for substantial growth, driven by advancements in personalized healthcare . Oncology and hematology, for instance, are major application areas where nano-enabled products like liposomal doxorubicin (e.g., Doxil) have already made significant contributions .
For generic developers, nanotechnology is not just another formulation technique; it is a powerful enabler for creating “super generics” or “value-added generics.” These are products that offer improved therapeutic profiles—such as better bioavailability, reduced side effects, or targeted delivery—that go beyond simple bioequivalence to the original brand. This allows generic manufacturers to differentiate their products in a crowded market and potentially qualify for regulatory pathways that offer periods of limited competition, thereby securing a more profitable market position . It represents a strategic shift where generic companies compete not just on price, but on enhanced therapeutic value.
3D Printing: Personalized Medicine and On-Demand Manufacturing
Three-dimensional (3D) printing, an advanced additive manufacturing technology, is poised to revolutionize pharmaceutical manufacturing by enabling unprecedented control over drug product characteristics and facilitating personalized medicine.
3D printing allows for the creation of customized medications with precise control over drug dosage, shape, size, and release profile . This technology can produce complex drug geometries that are simply unachievable using conventional manufacturing methods . This capability opens new avenues for tailoring treatments to individual patient needs.
Key applications of 3D printing in pharmaceuticals include:
- Personalized Medicine: The ability to customize dosage and release profiles on demand means medications can be tailored to individual patient requirements, which is particularly beneficial for populations with unique needs, such as pediatric or geriatric patients, or those requiring specific multi-drug combinations .
- Rapid Prototyping: 3D printing significantly accelerates the research and development phases by allowing for the rapid design and production of prototype drug formulations, streamlining the experimental process .
- On-Demand Manufacturing: This technology enables decentralized, on-demand drug production in hospitals and pharmacies, potentially reducing wait times, addressing logistical limitations, and improving access to medications, especially for orphan drugs or treatments for rare conditions that are not feasible to produce on a large scale .
A significant regulatory milestone occurred in August 2015 when the FDA approved SPRITAM® (levetiracetam) for oral use, the first 3D-printed drug product . Manufactured using Aprecia’s proprietary ZipDose® Technology, SPRITAM® is designed to rapidly disintegrate with just a sip of liquid, addressing a critical need for patients who struggle with swallowing traditional pills, such as those with dysphagia . This approval demonstrated the viability of additive manufacturing for commercial-scale pharmaceutical production.
While still emerging, 3D printing holds transformative potential for generic drug development. It is a powerful disruptor for niche markets and patient-centric generics. It promises to decentralize manufacturing, moving away from traditional large-batch models towards agile, on-demand production. This offers generic companies new opportunities to serve underserved patient populations or to develop products that overcome unique administration challenges, providing a significant competitive edge beyond traditional volume-based markets.
Navigating the Regulatory Landscape for Generic Approval
The journey of a generic drug to market is fundamentally governed by a complex and rigorous regulatory landscape, designed to ensure safety, efficacy, and quality. Understanding and strategically navigating these pathways is as crucial as the scientific formulation itself.
The Abbreviated New Drug Application (ANDA) and Marketing Authorisation Application (MAA)
The primary regulatory pathways for generic drug approval are the Abbreviated New Drug Application (ANDA) in the United States and the Marketing Authorisation Application (MAA) in the European Union. These processes, while “abbreviated” compared to new drug applications, are anything but simple.
Key Requirements for Submission and Approval
In the United States, the Abbreviated New Drug Application (ANDA) is the regulatory submission pathway for generic drug approval. It allows manufacturers to bring a generic drug to market without conducting extensive clinical trials for safety and efficacy, relying instead on the prior findings of the Reference Listed Drug (RLD) . However, this abbreviation does not imply a lack of rigor. A successful ANDA submission requires comprehensive data demonstrating therapeutic equivalence to the RLD, including:
- Product Sameness: The generic drug must be identical to the RLD in terms of active ingredients, conditions of use, method of administration, dosage form, strength, and labeling (with minor permissible modifications) .
- Bioavailability and Bioequivalence (BE) Data: This is paramount, proving that the generic drug delivers the same therapeutic effect as the RLD by demonstrating comparable rate and extent of absorption .
- Chemistry, Manufacturing, and Controls (CMC) Information: Detailed data on the drug’s formulation and composition, the manufacturing process, quality control measures, and comprehensive stability testing reports are essential to ensure consistent quality .
- Good Manufacturing Practices (cGMP) Compliance: The manufacturing facility must adhere to strict cGMP standards, often subject to pre-approval and routine inspections by the FDA .
- Patent and Exclusivity Certifications: Applicants must address any unexpired patents or regulatory exclusivities listed for the RLD, often through specific certifications (e.g., Paragraph IV) that can trigger litigation .
In the European Union, the equivalent process is the Marketing Authorisation Application (MAA). A centralized MAA, issued by the European Commission, grants permission to market a medicinal product throughout the European Economic Area (EEA) . For generics, the EMA assesses applications if the reference medicine was centrally authorized or if the generic medicine provides a significant therapeutic, scientific, or technical innovation or is in the interest of patients at the Union level .
The regulatory review process for an ANDA in the U.S. typically takes around 30 months, though applications for priority generics (e.g., drugs addressing shortages) may be expedited . The FDA’s review involves a multi-phase assessment of bioequivalence and safety data, labeling compliance, and manufacturing site inspections .
This entire submission process represents a complex, multi-stage regulatory “gauntlet” with significant financial implications. The high filing fees (e.g., $252,453 for an ANDA in 2024, set to increase to $321,920 in 2025) underscore the need for meticulous preparation . The risk of the FDA refusing to accept a filing due to deficiencies, resulting in the loss of a portion of this fee, highlights the critical importance of a complete and accurate electronic Common Technical Document (eCTD) submission . This transforms the regulatory process into a high-stakes financial endeavor, demanding not only scientific excellence but also robust regulatory affairs capabilities and strategic planning to navigate efficiently and avoid costly setbacks.
Pre-ANDA/MAA Interactions: Proactive Engagement with Regulators
Given the complexity and high stakes of generic drug submissions, proactive engagement with regulatory bodies is not merely a courtesy but a critical strategic imperative.
Sponsors are strongly encouraged to engage with regulatory agencies through pre-submission meetings or consultations, particularly for complex generics . For Abbreviated New Drug Applications (ANDAs) in the U.S., applicants can request Product-Specific Guidance (PSG) teleconferences. These meetings are particularly valuable if a company has already commenced an in vivo bioequivalence study, allowing them to obtain Agency feedback on the potential impact of new or revised PSGs on their development program .
This proactive regulatory dialogue serves as a powerful de-risking strategy. Early engagement allows generic developers to align their formulation and testing strategies with the latest regulatory expectations, clarify complex scientific questions (especially for novel or complex products), and potentially shorten approval timelines by avoiding common pitfalls and multiple review cycles . The case of generic diclofenac sodium topical gel, 1%, serves as a compelling example: the applicant utilized the FDA’s pre-ANDA Program to obtain Agency feedback on their proposed alternative bioequivalence (BE) approach, which was then incorporated into their original submission. This proactive engagement directly contributed to the drug’s approval in the first review cycle of its ANDA . This demonstrates how strategic regulatory dialogue can transform the regulatory body from a gatekeeper into a collaborative partner, accelerating market access and optimizing development resources.
Product-Specific Guidances (PSGs): FDA and EMA Directives
Regulatory agencies play a pivotal role in guiding generic drug development through the issuance of clear, product-specific directives. These guidances are not just compliance documents; they are strategic roadmaps for generic companies.
Tailored Pathways for Complex Generics
The FDA actively publishes Product-Specific Guidances (PSGs) to assist the generic pharmaceutical industry in identifying the most appropriate methodologies for developing drugs and generating the evidence needed to support Abbreviated New Drug Application (ANDA) approval . These PSGs are particularly valuable as they offer tailored pathways, especially for the more challenging complex generic drug products .
PSGs articulate the agency’s current thinking and expectations on how to develop generic drug products for specific Reference Listed Drugs (RLDs) . Recognizing the inherent difficulties in developing complex generics, the FDA has committed to issuing guidance for these drugs at least two years in advance of the first potential generic entrant. This forward-looking approach aims to provide generic firms with ample time to plan their development strategies effectively .
These PSGs are more than just regulatory documents; they are strategic roadmaps that can significantly de-risk and accelerate complex generic development. By providing clear, scientifically sound guidance on bioequivalence methods and the required data, PSGs help generic companies efficiently allocate their resources, avoid costly missteps, and navigate the intricate development process with greater certainty. This can potentially save years of development time, as industry representatives have indicated that the existence of a PSG can significantly shorten development timelines, especially for complex generic drugs .
The Role of ICH Guidelines in Global Harmonization
Beyond national guidances, international efforts to harmonize regulatory standards play a crucial role in facilitating global market access for generic drugs. The International Council for Harmonisation (ICH) is a key player in this endeavor.
ICH guidelines promote the global harmonization of technical scientific requirements for pharmaceuticals . While ICH guidelines do not directly dictate specific generic drug development pathways in all aspects, their foundational principles, particularly those related to quality (e.g., ICH Q8 on Pharmaceutical Development, Q9 on Quality Risk Management, and Q10 on Pharmaceutical Quality System), are instrumental for the effective implementation of Quality by Design (QbD) and for establishing overall quality standards across the industry .
The importance of a harmonized, international approach to regulatory standards for developing and approving complex generics is widely recognized within the industry, with over 95% of respondents in a survey agreeing on its significance . This consensus stems from a practical reality: divergent national requirements can significantly impede submissions and increase development costs for generic companies seeking to market their products across multiple countries .
The pursuit of global regulatory harmonization, often guided by ICH principles and bilateral initiatives (like the FDA-EMA Parallel Scientific Advice pilot program for complex generics), is therefore crucial for generic companies aiming for a global footprint. Harmonization streamlines approval processes across jurisdictions, reducing redundant testing and administrative burdens. This, in turn, facilitates broader patient access to affordable medicines and enhances market penetration for generic manufacturers, ultimately benefiting global public health .
Regulatory Science Initiatives: Fostering Innovation and Efficiency
Regulatory bodies are not merely passive gatekeepers; they are increasingly active participants in fostering innovation and efficiency within the generic drug industry. This proactive stance, particularly through dedicated regulatory science programs, is vital for addressing the evolving complexities of generic development.
Regulatory science programs, such as the FDA’s Generic Drug User Fee Amendments (GDUFA) Science and Research Program, are specifically designed to address the scientific challenges that often delay patient access to complex generics . These initiatives aim to actively foster the development, assessment, and approval of these harder-to-develop products .
The FDA, for instance, has made significant investments in research to develop new in vitro (laboratory-based), in silico (computational modeling), and in vivo (clinical) approaches. The goal is to establish bioequivalence for complex products more efficiently, thereby reducing the time and cost associated with traditional clinical studies . This includes supporting alternative bioequivalence (BE) approaches for challenging drug categories, such as those with narrow therapeutic indices, highly variable drugs, and products with partial area under the curve (pAUC) requirements .
These efforts demonstrate a crucial shift: regulatory bodies are acting as catalysts for generic innovation. Rather than simply reviewing submissions, they are proactively investing in scientific research and developing new methodologies (e.g., advancing PBPK modeling, refining in vitro BE approaches) to enable and accelerate the development and approval of complex generics. This proactive stance is fundamental for public health, ensuring continued access to affordable medicines, and for the economic viability of the generic industry. It fosters a collaborative ecosystem where scientists from regulatory agencies and industry can work together to modernize the generic value chain, leveraging quantitative methods and modeling (QMM) in pre-ANDA interactions and ANDA submission packages to shorten development timelines and reduce costs .
Strategic Advantage Through Intellectual Property and Competitive Intelligence
In the highly competitive generic pharmaceutical market, scientific prowess must be complemented by astute legal and business strategies. Understanding and leveraging intellectual property (IP) landscapes, coupled with robust competitive intelligence, can provide a decisive strategic advantage.
Patent Landscape Analysis and Freedom-to-Operate (FTO): Avoiding Pitfalls
The intellectual property (IP) rights surrounding innovator drugs, primarily patents and regulatory exclusivities, are designed to grant exclusive market periods and can significantly deter or delay generic entry . These patents can cover a wide array of aspects, including the chemical compounds themselves, specific formulations, methods of using the product, manufacturing processes, and even different crystalline forms (polymorphs) of the active pharmaceutical ingredient .
Identifying and Mitigating Patent Risks
Navigating this complex web of IP is paramount for generic developers. A Freedom-to-Operate (FTO) analysis is a critical legal assessment performed to identify any potential patent barriers that could prevent a generic product from being developed, manufactured, or commercialized without infringing on the intellectual property rights of others . This involves meticulously scrutinizing existing patents related to the drug compound, its formulations, manufacturing methods, and therapeutic uses .
The consequences of overlooking a patent are severe. An inadvertent infringement can lead to costly and protracted lawsuits, potentially forcing product withdrawals from the market, causing irreparable damage to a company’s reputation, and resulting in significant loss of market share . As Dr. Jane Smith, a patent attorney specializing in pharmaceutical law, aptly puts it: “An FTO search is not just a legal formality; it’s a strategic imperative. It’s the compass that guides pharmaceutical companies through the minefield of existing patents” .
The concept of a “patent thicket”—where a single drug product is protected by multiple, overlapping patents—is a formidable strategic barrier for generic entry . A comprehensive FTO analysis is, therefore, not merely a legal exercise but a crucial competitive intelligence endeavor. It allows companies to meticulously map these thickets, identify potential infringements, and, critically, devise “design-around” strategies (e.g., altering the drug’s chemical structure, developing a new formulation, or creating a novel delivery method that avoids patent claims) or explore licensing opportunities . This proactive approach is essential for de-risking market entry and ensuring a clear path to commercialization.
The Importance of Early FTO Analysis
The timing of a Freedom-to-Operate (FTO) analysis is as critical as its execution. For maximum strategic benefit, FTO analysis should be performed at the earliest meaningful stage in the generic development lifecycle, ideally as soon as the active pharmaceutical ingredient (API) to be repurposed, the new therapeutic target indication, and potentially the intended dose and formulation have been defined .
Conducting FTO analysis early offers several significant advantages:
- Avoiding Late-Stage Roadblocks: It helps identify and mitigate potential patent roadblocks before substantial resources are invested in development, preventing costly dead ends .
- Strategic Alignment: It allows for the alignment of stability protocols and other development strategies with target market regulations from the outset .
- Monitoring Evergreening: Early and continuous monitoring of the patent landscape enables generic companies to anticipate and adapt to “evergreening” strategies employed by innovator companies (where new patents are sought on minor modifications to extend market exclusivity) .
FTO analysis, when conducted early in the generic development lifecycle, serves as a critical driver of “smart” R&D investment. By proactively identifying less crowded patent spaces or areas where design-around strategies are feasible, companies can avoid wasting valuable R&D resources on products that face insurmountable legal barriers. This allows them to strategically pivot towards opportunities with a clearer and more predictable path to market, thereby optimizing resource allocation, accelerating time-to-market, and ultimately enhancing the overall return on investment for their generic portfolio .
Paragraph IV Certifications: Challenging Patents, Gaining Exclusivity
The Hatch-Waxman Act of 1984, a landmark piece of legislation in the U.S., fundamentally reshaped the generic drug landscape by creating an abbreviated approval pathway while also incentivizing generic companies to challenge existing patents.
The 180-Day Exclusivity Incentive
A pivotal provision of the Hatch-Waxman Act actively encourages generic companies to challenge existing patents through what is known as a “Paragraph IV certification” . When a generic manufacturer files an Abbreviated New Drug Application (ANDA), it must certify against any patents listed in the FDA’s Orange Book for the Reference Listed Drug (RLD). A Paragraph IV certification specifically states that the generic product either does not infringe on the listed patents or that those patents are invalid or unenforceable .
The significant incentive embedded in this process is the 180-day period of market exclusivity. If a generic company is the first to file an ANDA with a Paragraph IV certification and subsequently prevails in the ensuing patent infringement lawsuit (either through a court decision or a settlement), that company is granted a 180-day period during which it is the only generic version of that drug allowed on the market .
This exclusivity is a powerful economic driver. For six months, the first-filer can price its product slightly below the branded version, capturing a substantial share of the market before other generic competitors are permitted to enter and further erode prices . This creates an intense “race to file” among generic manufacturers. Being the first-filer is a strategic imperative that can yield significant, albeit temporary, market dominance and substantial profits, making early patent analysis, aggressive litigation strategies, and swift regulatory navigation critical components of a generic company’s business model .
Strategic Litigation and Settlement Considerations
The Paragraph IV certification process, while offering lucrative incentives, also plunges generic manufacturers into the complex world of patent litigation, necessitating careful strategic considerations.
Upon receiving a Paragraph IV certification, the branded innovator company has 45 days to file a patent infringement action against the generic applicant. If a lawsuit is filed within this window, it triggers an automatic 30-month stay on the FDA’s final approval of the generic application, allowing the patent holder time to litigate their patent rights .
Challenging patents through Paragraph IV certifications has become a routine and increasingly common part of doing business for generic companies, with a steady uptick in such cases over the years . However, this is a dual-edged sword. While the potential reward of 180-day exclusivity is substantial, the litigation process itself involves significant legal costs, considerable time investment, and the inherent risks of prolonged court battles. These factors can be a deterrent, particularly for smaller generic firms with limited legal and financial resources.
This complexity often leads to strategic settlements between innovator and generic companies. These settlements can take various forms, including:
- “Pay-for-delay” agreements: Where the innovator company pays the generic company to delay its market entry . These agreements have been controversial and are subject to antitrust scrutiny.
- Authorized generics (AGs): Where the innovator company licenses its product to a generic manufacturer (often its own subsidiary) to launch a generic version during or immediately after the 180-day exclusivity period, thereby capturing a share of the generic market .
These strategic considerations in litigation and settlement profoundly shape market entry dynamics and the competitive landscape for generic drugs. Generic companies must carefully weigh the potential rewards of exclusivity against the costs and risks of litigation, often finding that a negotiated settlement offers a more predictable, albeit potentially less lucrative, path to market.
Leveraging Patent Data for Competitive Intelligence: Insights from DrugPatentWatch
In the fast-paced and highly competitive generic pharmaceutical market, timely and accurate information is power. Patent data, when effectively leveraged, transforms into invaluable competitive intelligence, guiding strategic decisions and uncovering lucrative opportunities.
Market Entry Forecasting and Portfolio Management
For generic pharmaceutical companies, understanding precisely when patents on key drugs are set to expire is not just a matter of legal compliance; it unlocks lucrative commercial opportunities . The ability to accurately forecast these “patent cliffs” and have a bioequivalent alternative ready for launch at precisely the right moment is a cornerstone of success in the generic industry .
This is where specialized tools and platforms become indispensable. Resources like DrugPatentWatch provide comprehensive global biopharmaceutical business intelligence. They assist companies in identifying and evaluating commercial opportunities, accurately forecasting branded and generic drug pipelines, and anticipating future revenue events associated with patent expirations and generic entries .
Patent data, meticulously tracked and analyzed, thus serves as a powerful “crystal ball” for generic manufacturers. It allows them to predict market entry windows with greater precision, manage their product portfolios strategically, and prioritize their research and development (R&D) investments towards drugs nearing patent expiry. This proactive approach ensures that resources are allocated to the most promising targets, thereby maximizing their return on investment and securing a competitive edge in the market . It’s about transforming raw legal data into actionable business strategy.
Identifying Low-Competition Opportunities
Beyond simply tracking patent expirations, sophisticated competitive intelligence tools enable generic companies to identify lucrative niches within the market—specifically, low-competition opportunities.
DrugPatentWatch, for example, helps companies not only identify generic drug entry opportunities but also track investigational drugs and explore new indications for existing drugs, which can uncover unexpected market potential . Crucially, it assists in assessing the existing levels of generic competition for a given drug and identifying markets that are less crowded. This is particularly valuable for complex drugs, such as injectables, biologics, or those with intricate delivery systems (like inhalers), which require specialized manufacturing capabilities and thus deter many potential competitors .
This data-driven approach allows for a strategic shift from high-volume, low-margin simple generics to higher-value, less contested complex generics, thereby maximizing profitability and market share . Consider the success stories: Teva Pharmaceutical Industries launched a generic EpiPen in 2019, capitalizing on limited competition in the epinephrine auto-injector market and quickly capturing over 30% market share . Similarly, Mylan’s 2018 launch of generic Advair Diskus targeted a niche with few competitors, leveraging precise timing and market analysis to dominate sales . These examples underscore the power of competitive intelligence, particularly the granular insights derived from patent data, in shaping successful business strategies and securing profitable market positions in the generic pharmaceutical landscape.
Case Studies: Triumphs in Generic Formulation
The theoretical challenges and innovative solutions discussed thus far are best illuminated through real-world examples. These case studies demonstrate how generic pharmaceutical companies have successfully navigated complex formulation hurdles, bringing affordable, high-quality medicines to patients.
Success Stories in Modified-Release Drug Development
Modified-release (MR) formulations, designed for controlled drug delivery, present significant formulation complexities. Yet, several generic companies have achieved remarkable success in replicating these intricate systems.
Examples of Successful MR Generics:
- Generic Concerta (methylphenidate): Concerta is an osmotic-pressure controlled extended-release system. While some early generic versions faced significant dissolution issues, including “dose dumping” (releasing too much drug too quickly), the eventual development of successful generics for this complex osmotic system demonstrates the overcoming of substantial formulation hurdles . This required a deep understanding of the osmotic pump mechanism and careful replication of its release profile.
- Paliperidone palmitate extended-release injectable suspension (generic Invega Sustenna): In 2021, the FDA approved the first generic version of this long-acting injectable. The applicant successfully leveraged advanced modeling and simulation approaches for pharmacokinetic (PK) study designs and bioequivalence (BE) evaluations . This case highlights the increasing utility of sophisticated computational tools in navigating the complexities of advanced MR injectables.
Key Strategies Employed:
- Quality by Design (QbD): Successful MR generic developers rigorously implement QbD principles. This involves a systematic approach to understand the critical factors governing the drug’s in vivo performance and establishing a robust “design space” within which the product can be consistently manufactured to ensure bioequivalence .
- Modeling and Simulation: The use of advanced PK modeling is a game-changer. It guides the selection of optimal formulations and allows for the precise optimization of drug release profiles within a defined design space, significantly shortening development timelines and reducing the need for extensive, costly clinical trials . For example, by using PK modeling, companies can test a range of formulations in silico and select the most promising candidates for human studies, reducing the number of experimental prototypes needed.
- Advanced Polymer Science: Strategic selection and precise control of polymers are fundamental to MR success. Polymers are chosen not only for their ability to achieve sustained or delayed release but also for their capacity to deliver APIs to specific gastrointestinal target areas, depending on the drug’s physicochemical properties and the desired absorption window .
- Patient Compliance Focus: A key driver for MR formulations is improved patient convenience through infrequent dosing. Successful generic MR products are designed with this in mind, recognizing that enhanced patient adherence is a significant therapeutic and commercial benefit .
The successful development of modified-release generics is a powerful testament to the integration of advanced polymer science, sophisticated analytical techniques, and predictive computational modeling. It demonstrates that even the most complex physiological interactions—such as those governing drug release in the dynamic gastrointestinal environment—can be effectively managed through a deep understanding of formulation science and proactive regulatory engagement. This integrated scientific approach ultimately leads to products that not only meet stringent bioequivalence standards but also offer improved patient outcomes and significant market success.
Breakthroughs in Parenteral and Complex Injectable Generics
Parenteral and complex injectable drugs represent some of the highest-value and most challenging targets for generic development due to their stringent sterility requirements and intricate delivery systems. Yet, the industry has seen significant breakthroughs.
Examples of Successful Parenteral/Injectable Generics:
- Ferumoxytol injection (generic Feraheme): In 2021, the FDA approved the first complex generic for ferumoxytol, a parenteral iron product used to treat iron deficiency anemia. This approval was a direct result of scientific investment in meticulous product characterization and advanced bioequivalence (BE) study designs .
- Enoxaparin sodium for injection (generic Lovenox): This complex injectable, an anticoagulant, has been successfully genericized and is available in various prefilled syringe strengths, showcasing the ability to replicate complex biological mixtures and delivery formats .
- Apomorphine hydrochloride pen injection (generic Apokyn): The first generic version of this complex drug-device combination product, used for Parkinson’s disease, received FDA approval in February 2022 .
- Doxorubicin Hydrochloride Liposome Injection: In 2024, Lupin launched a generic equivalent of Baxter’s Doxil, a liposomal formulation of doxorubicin used in cancer therapy . This demonstrates successful genericization of a complex nanomedicine.
Key Strategies Employed:
- Advanced Delivery Systems: Successful generic parenteral developers leverage novel parenteral products such as nanoparticles, liposomes, microspheres, and emulsions. These advanced delivery systems are designed to improve pharmacokinetic/pharmacodynamic (PK/PD) behavior, enable reduced dosing frequency, and minimize adverse effects .
- Uncompromising Sterile Manufacturing Expertise: Investing heavily in specialized facilities and implementing stringent quality control measures are non-negotiable to meet the absolute sterility and pyrogenicity requirements of injectables . This includes advanced aseptic manufacturing systems and real-time endotoxin detection.
- Reformulation for Convenience and Compliance: Strategic reformulation efforts focus on enhancing user-friendliness and reducing administration errors. This includes converting lyophilized (freeze-dried) products into stable liquid forms, eliminating the need for reconstitution, or developing pre-filled syringes and auto-injectors that simplify self-administration for patients .
- Nanoparticle Systems: The incorporation of active pharmaceutical ingredients (APIs) into nanoparticle systems has proven effective in reducing side effects and, in some cases, enabling the use of drugs at higher levels than previously approved, by encapsulating the active compound and preventing its accumulation in or contact with cells/tissues that cause toxic side effects .
The successful genericization of complex injectables highlights a strategic shift towards higher-value products within the generic market. While the development costs are inherently higher due to stringent sterility requirements, the complexities of formulation, and the need for specialized delivery systems, the reduced competition and significant market potential make these products attractive targets for companies possessing advanced manufacturing capabilities and robust R&D expertise . This segment represents a strategic opportunity for generic companies to move up the value chain, differentiating themselves through advanced formulation and manufacturing rather than solely on price, thereby contributing to a more diversified and resilient generic market.
Innovations in Topical, Ophthalmic, and Inhalation Generics
Localized drug delivery systems, while seemingly simpler than systemic ones, present their own unique formulation challenges. Yet, generic companies have demonstrated significant innovation in these areas.
Examples of Successful Topical/Ophthalmic/Inhalation Generics:
- Diclofenac sodium topical gel, 1%: The FDA approved a generic version of this topical gel using physiologically based pharmacokinetic (PBPK) modeling and simulation to support an alternative bioequivalence (BE) approach, thereby avoiding a costly comparative clinical endpoint study . This case vividly demonstrates the power of computational modeling in streamlining topical generic development.
- Cyclosporine ophthalmic emulsion, 0.05% (referencing Restasis): In 2022, the FDA approved the first generic version of this complex ophthalmic emulsion, a significant achievement given the formulation’s intricate structure and the challenges of ocular delivery .
- Difluprednate ophthalmic emulsion (generic Durezol): The first generic of this ophthalmic emulsion was approved by the FDA using a novel in vitro BE approach, showcasing advancements in non-clinical assessment methods .
- Latanoprost ophthalmic solution (generic Xalatan/Lumigan): Generic versions of these glaucoma medications have been successfully launched, demonstrating equivalent safety and tolerability to their brand-name counterparts. Furthermore, their lower cost has been shown to improve medication adherence among patients, highlighting a direct patient benefit from generic entry .
- Generic EpiPen (epinephrine auto-injector): In 2019, Teva Pharmaceutical Industries successfully launched a generic EpiPen, capitalizing on limited competition in the complex epinephrine auto-injector market . This was a major win for patients needing affordable access to this life-saving drug-device combination.
- Generic Advair Diskus (fluticasone propionate/salmeterol inhalation powder): Mylan’s 2018 launch of a generic Advair Diskus successfully targeted a niche market with few competitors, leveraging precise timing and market analysis to achieve significant sales dominance . This was a triumph in replicating a complex dry powder inhaler.
Key Strategies Employed:
- Advanced Bioequivalence (BE) Approaches: Generic developers are increasingly utilizing a combination of in vitro, in silico (e.g., PBPK modeling), and novel clinical study designs to demonstrate equivalence efficiently, moving beyond traditional, often burdensome, comparative clinical trials .
- Formulation Optimization for Local Action: For topical and ophthalmic products, strategies are highly tailored to overcome specific biological barriers. This includes employing viscosity enhancers (e.g., polymers) to increase drug retention, permeation enhancers (e.g., surfactants, prodrugs) to improve penetration, and nanosuspensions to enhance solubility and bioavailability .
- Meticulous Device-Specific Development: For inhalation products, the drug-device combination is critical. Successful development involves meticulous engineering of both the formulation and the device to ensure consistent aerosol properties, particle size distribution, and delivered dose, which are paramount for effective pulmonary delivery .
- Strategic Market Entry: Companies employ sophisticated competitive intelligence, leveraging patent data to identify low-competition opportunities and time their market entry precisely, maximizing the commercial impact of their generic launches .
The success stories in topical, ophthalmic, and inhalation generics underscore a crucial principle: the need for highly tailored formulation and bioequivalence strategies. Unlike systemic drugs, where systemic exposure is the primary metric, these localized delivery systems demand a nuanced understanding of specific anatomical barriers, drug-device interactions, and the unique physiological environment of the target site. This pushes generic developers towards specialized expertise and innovative analytical methods, demonstrating that overcoming these challenges requires a bespoke approach for each complex product.
Lessons Learned: Navigating Past Challenges
The journey of generic drug development is a continuous learning process. Reviewing past challenges and failures offers invaluable insights that can guide future strategies and enhance success rates.
Several critical lessons have emerged from the industry’s experience in overcoming formulation hurdles:
- The Importance of Robust and Accurate Data: The FDA’s scrutiny of submitted data is exceptionally thorough. Incomplete, inaccurate, or poorly substantiated submissions can lead to “refuse-to-receive” letters, multiple review cycles, or significant delays in approval . This emphasizes that data integrity and scientific rigor are non-negotiable.
- The Critical Impact of Excipient Quality and Interaction: Even seemingly minor differences in inactive ingredients or the presence of impurities within excipients can lead to profound formulation issues or bioequivalence failures. Historical cases have shown generic drugs dissolving at different rates or containing contaminants, leading to inconsistent doses or adverse effects . This highlights that excipient selection must go beyond basic compliance to include rigorous compatibility studies and supplier qualification.
- The Need for Continuous Monitoring of Stability: Drug stability is not a one-time test performed at the end of development. It must be continuously monitored throughout the entire product lifecycle, from initial formulation and manufacturing to commercial distribution and storage . Unforeseen degradation pathways or polymorphic transformations can emerge over time, necessitating ongoing vigilance.
- Regulatory Evolution Necessitates Ongoing Engagement: Regulatory bodies, particularly for complex generics, are continuously evolving their guidelines and expectations. What was acceptable yesterday may not be tomorrow. This necessitates ongoing proactive engagement with regulators, continuous monitoring of product-specific guidances (PSGs), and a willingness to adapt manufacturing and testing strategies to new scientific standards .
The lessons from these past challenges highlight a crucial evolution in the generic pharmaceutical industry: a profound shift from reactive problem-solving to proactive, risk-managed development. This involves a strategic investment in early-stage characterization, predictive modeling, and robust quality systems to anticipate and mitigate formulation and manufacturing challenges before they escalate into costly failures. This embodies the true spirit of Quality by Design (QbD), where quality is built into the product and process from the outset. By internalizing these lessons, generic companies can enhance their efficiency, reduce risks, and consistently bring high-quality, affordable medicines to patients.
The Future of Generic Drug Formulation: Trends, Opportunities, and Outlook
The landscape of generic drug formulation is dynamic, constantly reshaped by technological advancements, evolving regulatory expectations, and a growing emphasis on patient-centric solutions. The future promises even greater sophistication and strategic opportunities.
Emerging Technologies and Their Transformative Potential
The trajectory of generic drug formulation is undeniably “tech-driven,” moving beyond simple chemical replication to embrace revolutionary manufacturing processes, AI-powered design, and novel delivery systems. These technologies are not merely incremental improvements; they represent a fundamental shift that will enable generics to tackle increasingly complex drugs, offer enhanced patient benefits, and reshape competitive dynamics.
- Continuous Manufacturing: This represents a paradigm shift from traditional batch manufacturing processes in pharmaceutical production. Unlike batch processing, which involves discrete steps and interruptions, continuous manufacturing integrates all production steps into a seamless, uninterrupted flow . This approach offers significant advantages: it enhances product consistency, reduces batch failures, minimizes production variability, and dramatically improves efficiency and quality control by allowing real-time monitoring and adjustment.
- Advanced AI/Machine Learning in Formulation: The integration of Artificial Intelligence (AI) and Machine Learning (ML) is rapidly transforming pharmaceutical R&D. These technologies are increasingly used to analyze complex datasets (e.g., from high-throughput screening, stability studies, and bioequivalence data), predict drug behavior, optimize formulations, and streamline research and development workflows . By identifying hidden patterns and predicting outcomes, AI/ML can significantly reduce the need for extensive physical experiments, accelerating the identification of optimal formulations and minimizing costly iterations.
- Personalized Medicine via 3D Printing: Three-dimensional (3D) printing is poised to revolutionize pharmaceutical manufacturing by enabling true personalized medicine. This technology allows for precise control over drug dosage, shape, size, and release profile, making it possible to create customized medications tailored to individual patient needs . This includes developing formulations with improved palatability for children or the elderly, or combining multiple drugs into a single, patient-specific dosage form (polypharmacy). The FDA’s approval of SPRITAM® in 2015 demonstrated the commercial viability of this approach .
- Nanotechnology for Enhanced Delivery: Continued advancements in nanotechnology, particularly in the design of nanocarriers such as liposomes, dendrimers, and various nanoparticles, are expanding therapeutic horizons. These systems are engineered to enhance the solubility and bioavailability of poorly soluble APIs, provide targeted drug delivery to specific tissues or cells, and enable controlled or sustained drug release . This is particularly crucial for complex molecules and challenging indications like oncology, where precision and reduced side effects are paramount. Nanoparticles can significantly enhance the bioavailability and solubility of poorly soluble APIs, making them more effective and easier to administer .
These emerging technologies collectively represent a “tech-driven” generic revolution. They empower generic companies to move beyond simply replicating existing products at lower costs. Instead, they can tackle increasingly complex drugs, offer enhanced patient benefits (e.g., improved adherence, fewer side effects), and fundamentally reshape competitive dynamics by creating differentiated, value-added products.
Collaborative Ecosystems: Industry, Academia, and Regulatory Synergy
The escalating complexity of generic drug formulation, particularly for complex generics and biosimilars, necessitates a fundamental shift from siloed development to collaborative ecosystems. No single entity possesses all the expertise and resources required to navigate these intricate challenges alone.
The achievement of consistent quality and therapeutic performance, especially for complex drug delivery systems, increasingly demands close collaboration among industry, academia, and regulatory agencies . This synergy fosters a collective intelligence that can accelerate innovation and establish robust scientific standards.
For instance, the FDA actively encourages scientists in both industry and its own ranks to form an “ecosystem” to modernize the generic value chain. This involves leveraging quantitative methods and modeling (QMM) in pre-ANDA interactions and ANDA submission packages. Such collaborative efforts are designed to shorten development timelines and reduce costs by proactively addressing scientific questions and aligning strategies early .
This emphasis on collaborative ecosystems highlights the power of collective intelligence. By fostering open dialogue, sharing research findings, and engaging in joint problem-solving initiatives, the pharmaceutical industry can overcome barriers more efficiently. This synergy accelerates the development of innovative formulation strategies, streamlines regulatory pathways, and ultimately brings high-quality, affordable medicines to patients faster. It’s a recognition that the future of generic drug development lies in shared knowledge and mutual advancement.
Conclusion
The generic pharmaceutical industry, a cornerstone of affordable healthcare, stands at a pivotal moment. Far from being a realm of mere replication, it has evolved into a sophisticated arena demanding cutting-edge scientific expertise, strategic foresight, and an unwavering commitment to quality. The journey of a generic drug from patent expiry to patient access is a complex tapestry woven with physicochemical challenges, intricate regulatory pathways, and dynamic market forces.
We have explored how generic developers must meticulously match the performance of Reference Listed Drugs (RLDs), navigating the nuanced requirements of Q1/Q2/Q3 sameness, overcoming solubility and dissolution hurdles, controlling polymorphic forms, and ensuring long-term stability. The shift towards complex generics—encompassing modified-release formulations, parenterals, topicals, ophthalmics, inhalations, and the pinnacle of biosimilars—has further amplified these challenges, demanding specialized knowledge and innovative solutions tailored to each unique dosage form.
However, the industry is not merely reacting to these challenges; it is proactively pioneering solutions. The adoption of Quality by Design (QbD) and Process Analytical Technology (PAT) is transforming development from empirical trial-and-error to a knowledge-driven, real-time controlled process. Advanced analytical characterization provides unprecedented insights into drug products, while computational modeling and in silico prediction (including PBPK modeling and AI/ML) are accelerating development and reducing reliance on costly human trials. Novel drug delivery systems like nanotechnology are enabling “super generics” with enhanced therapeutic profiles, and 3D printing promises personalized, on-demand manufacturing.
Strategically, success hinges on a deep understanding of the intellectual property landscape. Meticulous Freedom-to-Operate (FTO) analyses are essential to navigate patent thickets and avoid costly litigation, while strategic Paragraph IV certifications offer lucrative exclusivity incentives. Leveraging competitive intelligence, often through platforms like DrugPatentWatch, allows companies to forecast market entry, manage portfolios, and identify low-competition opportunities, turning legal data into actionable business advantage.
Ultimately, the future of generic drug formulation is one of continuous innovation and collaboration. The increasing complexity of drug products, coupled with the relentless demand for affordability and access, necessitates a synergistic ecosystem where industry, academia, and regulatory bodies work hand-in-hand. By embracing advanced technologies, fostering proactive regulatory engagement, and cultivating a culture of scientific excellence, generic pharmaceutical companies can not only overcome formulation challenges but also continue their vital mission of delivering high-quality, affordable medicines to patients worldwide, shaping a healthier, more accessible future for all.
Key Takeaways
- Generics are Pillars of Affordability: Generic drugs significantly reduce healthcare costs (e.g., up to 85% price reduction with multiple competitors) and improve patient access to essential medicines.
- Bioequivalence is Key, Not Identicality: Generic drugs must be bioequivalent (same rate and extent of absorption) but can differ in excipients and manufacturing processes, creating complex formulation challenges.
- Complex Generics are the New Frontier: The industry is shifting focus to complex generics (e.g., modified-release, injectables, biologics, drug-device combinations) due to market saturation in simple generics and higher profit potential.
- Physicochemical Hurdles are Diverse: Challenges include achieving Q1/Q2/Q3 sameness, overcoming poor solubility and dissolution, controlling polymorphism, and ensuring long-term stability against various degradation pathways.
- Excipients are Active Players: Inactive ingredients (excipients) can significantly impact drug stability and performance, necessitating rigorous compatibility studies and a deep understanding of their potential interactions and impurities.
- Scale-Up is a “Valley of Death”: Translating lab-scale formulations to commercial production is challenging, with process variability and supply chain vulnerabilities (e.g., reliance on offshore APIs) posing significant risks.
- Dosage Forms Demand Tailored Solutions: Each specialized dosage form (MR, parenteral, topical, ophthalmic, inhalation, biosimilars) presents unique formulation complexities requiring bespoke scientific and regulatory approaches.
- QbD and PAT Drive Quality: Quality by Design (QbD) and Process Analytical Technology (PAT) are transforming development into a proactive, knowledge-driven process, enhancing quality, efficiency, and reducing risks.
- Advanced Analytics and Modeling are Essential: High-resolution analytical techniques, bioanalytical methods, and computational modeling (PBPK, in silico, AI/ML) are crucial for deep product understanding, predictive insights, and accelerating development.
- Novel Delivery Systems Offer Differentiation: Nanotechnology and 3D printing are enabling “super generics” with enhanced therapeutic profiles and personalized medicine, allowing generic companies to compete on value beyond price.
- Regulatory Navigation is Strategic: Proactive engagement with regulatory bodies (e.g., pre-ANDA meetings, leveraging PSGs) and meticulous intellectual property strategies (FTO, Paragraph IV certifications) are critical for de-risking and accelerating market entry.
- Collaboration is the Future: Overcoming the escalating complexity requires collaborative ecosystems among industry, academia, and regulatory agencies to foster innovation and ensure global access to affordable medicines.
FAQ Section
1. What is the fundamental difference between a generic drug and its brand-name counterpart, beyond price?
While a generic drug contains the same active pharmaceutical ingredient (API) as the brand-name drug and is therapeutically equivalent, meaning it produces the same clinical effect and safety profile, it is not an exact chemical clone in all respects . Generics may differ in inactive ingredients (excipients), manufacturing processes, color, taste, and packaging . The core difference lies in the development pathway: brand-name drugs undergo extensive clinical trials to prove safety and efficacy from scratch, whereas generics demonstrate “bioequivalence” to an already approved brand, proving their API is absorbed at the same rate and extent . This allows generics to be significantly more affordable, typically 80-85% cheaper than their brand-name versions .
2. How do “complex generics” differ from “simple generics,” and why are they becoming a strategic focus for manufacturers?
Simple generics typically refer to oral solid dosage forms (like tablets or capsules) that are relatively straightforward to replicate. Complex generics, on the other hand, involve intricate active pharmaceutical ingredients (e.g., peptides), sophisticated formulations (e.g., liposomes, nanoparticles), advanced dosage forms (e.g., modified-release, transdermal patches, inhalers), or drug-device combinations (e.g., auto-injectors) . They are strategically important because the market for simple generics is becoming saturated with razor-thin profit margins . Complex generics, while harder and more costly to develop, face significantly less competition, offering greater market potential and higher profitability for companies with the necessary scientific and manufacturing capabilities .
3. What role does “Quality by Design (QbD)” play in overcoming generic formulation challenges?
Quality by Design (QbD) is a proactive, systematic approach that builds quality into a drug product from the very beginning of its development, rather than relying solely on end-product testing . For generics, QbD helps overcome formulation challenges by systematically identifying and controlling critical quality attributes (CQAs), critical material attributes (CMAs), and critical process parameters (CPPs) . By using tools like risk assessment and Design of Experiments (DoE), QbD enables a deep understanding of how formulation and manufacturing variables impact product performance, thereby reducing variability, minimizing costly failures, and accelerating development timelines, ultimately leading to more robust and consistently high-quality generic drugs .
4. How do advanced technologies like nanotechnology and 3D printing contribute to generic drug development?
These advanced technologies are transforming generic drug development by enabling new levels of performance and customization. Nanotechnology allows for the engineering of drugs at the nanoscale, significantly enhancing the solubility and bioavailability of poorly soluble active pharmaceutical ingredients (APIs), providing targeted drug delivery, and enabling controlled release profiles . This allows for the creation of “super generics” with improved therapeutic benefits . 3D printing, or additive manufacturing, enables precise control over drug dosage, shape, size, and release profile, facilitating personalized medicine. It allows for on-demand production of customized medications, which is particularly beneficial for specific patient populations (e.g., pediatrics, geriatrics) or niche markets . Both technologies empower generic companies to differentiate their products beyond price competition.
5. How does patent data, such as that provided by DrugPatentWatch, offer a competitive advantage in generic drug development?
Patent data is a crucial source of competitive intelligence for generic drug developers. Platforms like DrugPatentWatch provide detailed information on patent expirations, allowing companies to accurately forecast market entry windows and strategically plan their product portfolios . Beyond simple expiry dates, patent landscape analysis (Freedom-to-Operate or FTO analysis) helps identify potential patent thickets and risks, guiding “design-around” strategies or licensing opportunities to avoid costly litigation . By leveraging this data, generic companies can identify lucrative low-competition opportunities, particularly for complex drugs, and time their market entry precisely to maximize market share and profitability, transforming legal information into a powerful business advantage .
References
- Wikipedia. Generic drug. Available at: https://en.wikipedia.org/wiki/Generic_drug. Accessed:.
- FDA. Drug Development Process. Available at: https://www.fda.gov/patients/learn-about-drug-and-device-approvals/drug-development-process. Accessed:.
- FDA. Bioequivalence. Available at: https://www.fda.gov/animal-veterinary/abbreviated-new-animal-drug-applications/bioequivalence. Accessed:.
- Merck Manuals. Bioequivalence and Interchangeability of Generic Drugs. Available at: https://www.merckmanuals.com/home/drugs/brand-name-and-generic-drugs/bioequivalence-and-interchangeability-of-generic-drugs. Accessed:.
- EMA. Product-specific bioequivalence guidance. Available at: https://www.ema.europa.eu/en/human-regulatory-overview/research-development/scientific-guidelines/clinical-pharmacology-pharmacokinetics/product-specific-bioequivalence-guidance. Accessed:.
- ResearchGate. International Guidelines for Bioequivalence of Systemically Available Orally Administered Generic Drug Products: A Survey of Similarities and Differences. Available at: https://www.researchgate.net/publication/244992555_International_Guidelines_for_Bioequivalence_of_Systemically_Available_Orally_Administered_Generic_Drug_Products_A_Survey_of_Similarities_and_Differences. Accessed:.
- Excedr. What Is Abbreviated New Drug Application. Available at: https://www.excedr.com/blog/what-is-abbreviated-new-drug-application. Accessed:.
- Vici Health Sciences. ANDA Checklist for Filing Process. Available at: https://vicihealthsciences.com/anda-checklist-for-filing-process/. Accessed:.
- Wikipedia. Marketing Authorisation Application. Available at: https://en.wikipedia.org/wiki/Marketing_Authorisation_Application. Accessed:.
- PMC. In principle, there are three procedures for submitting a Marketing Authorization Application (MAA) in the EU. Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC3149704/. Accessed:.
- Complex Generics. Q1/Q2 Sameness Assessments. Available at: https://www.complexgenerics.org/wp-content/uploads/crcg/prsnt-Kozak20200929-SBIA.pdf. Accessed:.
- FDA. What is Q1/Q2 Sameness? Available at: https://www.fda.gov/media/186015/download. Accessed:.
- BMJ. The FDA must determine, based on the data it receives, that the drug seems safe enough and that there is substantial evidence that the drug will have the effect it is represented to have. Available at: https://www.bmj.com/content/351/bmj.h4633. Accessed:.
- FDA. FY2015 Regulatory Science Research Report: Nanotechnology: Physiochemical Characterization of Nano-Sized Drug Products. Available at: https://www.fda.gov/industry/generic-drug-user-fee-amendments/fy2015-regulatory-science-research-report-nanotechnology-physiochemical-characterization-nano-sized. Accessed:.
- PMC. FDA Critical Path Initiatives: Opportunities for Generic Drug Development. Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC2751455/. Accessed:.
- DrugPatentWatch. Key Considerations for Generic Drug Stability Testing. Available at: https://www.drugpatentwatch.com/blog/key-considerations-for-generic-drug-stability-testing/. Accessed:.
- GSC Online Press. Stability and bioequivalence challenges in generic drug formulation: A regulatory perspective. Available at: https://gsconlinepress.com/journals/gscbps/sites/default/files/GSCBPS-2025-0189.pdf. Accessed:.
- Drug-Dev. SPECIAL FEATURE – Solubility & Bioavailability: Difficult Beasts to Tame. Available at: https://drug-dev.com/special-feature-solubility-bioavailability-difficult-beasts-to-tame/. Accessed:.
- ResearchGate. Drug Solubility: Importance and Enhancement Techniques. Available at: https://www.researchgate.net/publication/230566312_Drug_Solubility_Importance_and_Enhancement_Techniques. Accessed:.
- FDA. Dissolution Methods Database. Available at: https://www.fda.gov/drugs/drug-approvals-and-databases/dissolution-methods-database. Accessed:.
- PMC. Dissolution Testing for Generic Drugs: An FDA Perspective. Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC3160163/. Accessed:.
- ResearchGate. Polymorphism in generic drug product. Available at: https://www.researchgate.net/publication/8617390_Polymorphism_in_generic_drug_product. Accessed:.
- SciELO. Polymorphism: an evaluation of the potential risk to the quality of drug products from the Farmácia Popular Rede Própria. Available at: https://www.scielo.br/j/bjps/a/f35tDgLNrBb3jdyQBQNyggs/?format=pdf&lang=en. Accessed:.
- Wiley. Compendium of Drug Degradation Pathways. Available at: https://www.wiley.com/en-us/Compendium+of+Drug+Degradation+Pathways-p-00100194. Accessed:.
- Pharmatech Associates. 5 Forced Degradation Pathways To Detect Drug Instability. Available at: https://pharmatechassociates.com/blog/forced-degredation/. Accessed:.
- MDPI. Pharmaceutics | Special Issue : Drug Stability and Stabilization Techniques. Available at: https://www.mdpi.com/journal/pharmaceutics/special_issues/drug_stab_tech. Accessed:.
- PMC. Various techniques are used for the enhancement of the solubility of poorly soluble drugs. Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC3399483/. Accessed:.
- StudySmarter. Solubility enhancement. Available at: https://www.studysmarter.co.uk/explanations/medicine/pharmacy/solubility-enhancement/. Accessed:.
- Symmetric Events. Dissolution Testing, Biowaivers & Key Methods. Available at: https://www.symmetric.events/blog-dissolution-testing-biowaivers-key-methods-regulatory-insights/. Accessed:.
- New Drug Approvals. The US FDA requires submitters of an Abbreviated New Drug Application (ANDA – new generic) that they must include a polymorphism study in order to demonstrate equivalence. Available at: https://newdrugapprovals.org/27-2/. Accessed:.
- All For Drugs. DRUG POLYMORPHISM. Available at: https://www.allfordrugs.com/polymorphism/. Accessed:.
- JoCPR. The Impact of Formulation Strategies on Drug Stability and Bioavailability. Available at: https://www.jocpr.com/articles/the-impact-of-formulation-strategies-on-drug-stability-and-bioavailability-10201.html. Accessed:.
- ResearchGate. Stabilization of Pharmaceuticals to Oxidative Degradation. Available at: https://www.researchgate.net/publication/11508246_Stabilization_of_Pharmaceuticals_to_Oxidative_Degradation. Accessed:.
- PharmaTech. Selection of Excipients in Generic Formulations. Available at: https://www.pharmtech.com/view/selection-excipients-generic-formulations. Accessed:.
- CASSS. CMC Challenges with Complex Formulations – Excipient Selection and Impact on Product Stability and Process Consistency. Available at: https://www.casss.org/docs/default-source/wcbp/2025-roundtable-notes/cmc-challenges-with-complex-formulations—excipient-selection-and-impact-on-product-stability-and-process-consistency.pdf?sfvrsn=14780b31_6. Accessed:.
- Pharma Excipients. Novel Vial-in-Vial. Available at: https://www.pharmaexcipients.com/news/novel-vial%E2%80%91in%E2%80%91vial/. Accessed:.
- PMC. Drug-excipient compatibility study (DECS) is one of the critical steps during pre-formulation studies. Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC10169295/. Accessed:.
- DrugPatentWatch. Top 10 Challenges in Generic Drug Development. Available at: https://www.drugpatentwatch.com/blog/top-10-challenges-in-generic-drug-development/. Accessed:.
- FDA. Generic Drug Approval Process. Available at: https://www.fda.gov/drugs/cder-conversations/generic-drug-approval-process. Accessed:.
- PharmExec. Tariffs Won’t Fix Weakened Drug Supply Chains. Available at: https://www.pharmexec.com/view/tariffs-wont-fix-weakened-drug-supply-chains. Accessed:.
- SynergBiopharma. Manufacturing Gaps US Pharmaceutical Industry Exposed by Tariff Wars. Available at: https://synergbiopharma.com/manufacturing-gaps-us-pharmaceutical-industry-exposed-by-tariff-wars/. Accessed:.
- Journal JPRI. Quality by Design (QbD) in Generic Solid Oral Drug Products. Available at: https://www.journaljpri.com/index.php/JPRI/article/view/7677. Accessed:.
- GSC Online Press. Stability and Bioequivalence Challenges in Generic Drug Formulation. Available at: https://gsconlinepress.com/journals/gscbps/sites/default/files/GSCBPS-2025-0189.pdf. Accessed:.
- PharmaNow. Parenteral Drugs Formulation Challenges. Available at: https://www.pharmanow.live/knowledge-hub/innovation/parenteral-drugs-formulation-challenges. Accessed:.
- PharmaTech. Addressing Parenteral Manufacturing Challenges. Available at: https://www.pharmtech.com/view/addressing-parenteral-manufacturing-challenges. Accessed:.
- FDA. Formulation, Characterization, and Cutaneous Pharmacokinetics to Facilitate Generic Topical Product. Available at: https://www.fda.gov/drugs/news-events-human-drugs/formulation-characterization-and-cutaneous-pharmacokinetics-facilitate-generic-topical-product. Accessed:.
- PMC. Systemic delivery of drugs from topical dosage forms has several problems. Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC3535108/. Accessed:.
- PMC. Innovative Formulation Strategies for Biosimilars. Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC12196224/. Accessed:.
- PharmaTech. Overcoming Biosimilar Scaling Challenges. Available at: https://www.pharmtech.com/view/overcoming-biosimilar-scaling-challenges. Accessed:.
- Celegence. Generic Inhalation Drug Development Regulatory Strategy Support. Available at: https://www.celegence.com/generic-inhalation-drug-development-regulatory-strategy-support/. Accessed:.
- OnDrugDelivery. Why Are Inhalation Devices So Difficult to Develop? Available at: https://www.ondrugdelivery.com/why-are-inhalation-devices-so-difficult-to-develop/. Accessed:.
- US Pharmacist. Ophthalmic Medications: The Safety and Efficacy of Brand-Name Versus Generic Formulations. Available at: https://www.uspharmacist.com/article/ophthalmic-medications-the-safety-and-efficacy-of-brandname-versus-generic-formulations. Accessed:.
- PMC. Low ocular bioavailability of topically applied drug molecules can considerably limit their efficacy. Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC7170772/. Accessed:.
- FDA. Guidances | Drugs. Available at: https://www.fda.gov/drugs/guidance-compliance-regulatory-information/guidances-drugs. Accessed:.
- FDA. Upcoming Product-Specific Guidances for Generic Drug Product Development. Available at: https://www.fda.gov/drugs/guidances-drugs/upcoming-product-specific-guidances-generic-drug-product-development. Accessed:.
- EMA. Generic and hybrid medicinal products eligibility can be granted to the Centralised procedure. Available at: https://www.ema.europa.eu/en/human-regulatory-overview/marketing-authorisation/generic-hybrid-medicines/generic-hybrid-applications. Accessed:.
- EMA. Generic and Hybrid Medicines. Available at: https://www.ema.europa.eu/en/human-regulatory-overview/marketing-authorisation/generic-hybrid-medicines. Accessed:.
- ICH. Reflection Papers. Available at: https://www.ich.org/page/reflection-papers. Accessed:.
- FDA. Product-Specific Guidances for Generic Drug Development. Available at: https://www.fda.gov/drugs/guidances-drugs/product-specific-guidances-generic-drug-development. Accessed:.
- JoCPR. A Brief Review on Analytical Techniques in Bioequivalence Studies. Available at: https://jcpr.in/index.php/journal/article/view/117. Accessed:.
- ResearchGate. A Brief Review on Analytical Techniques in Bioequivalence Studies. Available at: https://www.researchgate.net/publication/377798805_A_Brief_Review_on_Analytical_Techniques_in_Bioequivalence_Studies. Accessed:.
- Premier Research. CMC Perspectives of In Vitro-In Vivo Correlation (IVIVC) in Drug Development. Available at: https://premier-research.com/perspectives/cmc-perspectives-of-in-vitro-in-vivo-correlation-ivivc-in-drug-development/. Accessed:.
- WJARR. The pharmacological discipline of In vivo–In Vitro Correlation (IVIVC) plays a crucial role in drug development. Available at: https://wjarr.com/sites/default/files/WJARR-2024-1197.pdf. Accessed:.
- Congress. Intellectual property (IP) rights play an important role in the development and pricing of prescription drugs and biologics. Available at: https://www.congress.gov/crs-product/R46679. Accessed:.
- PhRMA. Intellectual Property. Available at: https://phrma.org/policy-issues/intellectual-property. Accessed:.
- FDLI. Patents are widely considered to be a critical incentive for drug development. Available at: https://www.fdli.org/wp-content/uploads/2022/08/7-Darrow-and-Mai.pdf. Accessed:.
- PMC. Ally Memedovich and colleagues report on patent challenges within the first year of eligibility among small-molecule drugs approved by the FDA from 2007-2018. Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC11867330/. Accessed:.
- DrugPatentWatch. Conducting a Biopharmaceutical Freedom-to-Operate (FTO) Analysis: Key Considerations for Generic Drug Stability Testing. Available at: https://www.drugpatentwatch.com/blog/conducting-a-biopharmaceutical-freedom-to-operate-fto-analysis-key-considerations-for-generic-drug-stability-testing/. Accessed:.
- DrugPatentWatch. How to Conduct a Drug Patent FTO Search. Available at: https://www.drugpatentwatch.com/blog/how-to-conduct-a-drug-patent-fto-search/. Accessed:.
- The FDA Law Blog. Go For It: Connect Paragraph IV. Available at: https://www.thefdalawblog.com/2019/06/go-for-it-connect-paragraph-iv/. Accessed:.
- ParagraphFour. Paragraph IV Explained. Available at: https://paragraphfour.com/paragraph-iv-explained/. Accessed:.
- DrugPatentWatch. Global Biopharmaceutical Business Intelligence. Available at: https://www.drugpatentwatch.com/. Accessed:.
- NBER. How Generic Drugs, Patents, and Price Controls Affect Markets. Available at: https://www.nber.org/digest/jan15/how-generic-drugs-patents-and-price-controls-affect-markets. Accessed:.
- PatentPC. How to Protect Intellectual Property Generic Drug Development. Available at: https://patentpc.com/blog/how-to-protect-intellectual-property-generic-drug-development. Accessed:.
- PatentPC. The Role of Patents in Biopharmaceutical Drug Formulation. Available at: https://patentpc.com/blog/role-of-patents-in-drug-formulation. Accessed:.
- ResearchGate. Computational Modelling for Formulation Design. Available at: https://www.researchgate.net/publication/378805_Computational_Modelling_for_Formulation_Design. Accessed:.
- FDA. Impact Story: Modeling Tools Could Modernize Generic Drug Development. Available at: https://www.fda.gov/drugs/regulatory-science-action/impact-story-modeling-tools-could-modernize-generic-drug-development. Accessed:.
- InSilicoMinds. Small Molecules. Available at: https://insilicominds.com/generic/. Accessed:.
- InSilicoMinds. Generics: Small Molecules. Available at: https://insilicominds.com/generics-small-molecules/. Accessed:.
- PMC. PBPK modeling can support relative BA and BE assessment in pediatrics. Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC12158840/. Accessed:.
- Biopharma Services. Bioequivalence PBPK Modeling in Predicting Drug Behavior. Available at: https://www.biopharmaservices.com/blog/bioequivalence-pbpk-modeling-in-predicting-drug-behavior/. Accessed:.
- Zimlab. Nanotechnology in Pharmaceutical Drug Delivery Systems: Revolutionizing Modern Medicine. Available at: https://www.zimlab.in/blog-posts/nanotechnology-in-pharmaceutical-drug-delivery-systems-revolutionizing-modern-medicine. Accessed:.
- StudySmarter. Nanotechnology in Pharmacy. Available at: https://www.studysmarter.co.uk/explanations/medicine/pharmacy/nanotechnology-in-pharmacy/. Accessed:.
- YouTube. 3D Drug Printing. Available at: https://www.youtube.com/watch?v=hEmrkHOf23Y. Accessed:.
- PharmaTech. Entering New Domains: 3D Printing Drug Products. Available at: https://www.pharmtech.com/view/entering-new-domains-3d-printing-drug-products. Accessed:.
- Precedence Research. Generic Drugs Market Size to Hit USD 728.64 Billion by 2034. Available at: https://www.precedenceresearch.com/generic-drugs-market. Accessed:.
- Custom Market Insights. Global Generic Drug Market Size was valued at USD 491.35 Billion in 2024. Available at: https://www.custommarketinsights.com/report/generic-drug-market/. Accessed:.
- MedShadow. Are Generic Drugs Safe? Available at: https://medshadow.org/are-generic-drugs-safe/. Accessed:.
- EMA. Guideline on the pharmacokinetic and clinical evaluation of modified release dosage forms. Available at: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-pharmacokinetic-and-clinical-evaluation-modified-release-dosage-forms_en.pdf. Accessed:.
- PMC. Challenges and Opportunities in Establishing Scientific and Regulatory Standards for Assuring Therapeutic Equivalence of Modified Release Products. Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC2895434/. Accessed:.
- FDA. Helpful Webinars and Other Resources for Generic Drug Manufacturers. Available at: https://www.fda.gov/drugs/abbreviated-new-drug-application-anda/helpful-webinars-and-other-resources-generic-drug-manufacturers. Accessed:.
- Roquette. Parenteral Preparations: Challenges in Formulation. Available at: https://www.roquette.com/innovation-hub/pharma/expert-opinion/parenteral-preparations-challenges-in-formulation. Accessed:.
- Frontiers in Pharmacology. In assessing topical generic formulations, the regulatory authorities request a demonstration of the pharmaceutical and therapeutic equivalence. Available at: https://www.frontiersin.org/journals/pharmacology/articles/10.3389/fphar.2024.1330712/full. Accessed:.
- PMC. For various reasons, the generic firm may choose to formulate the generic product with a different excipient composition to the brand product. Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC3691439/. Accessed:.
- PMC. Two important key takeaways emerge: (1) strategic innovation in formulation, especially using buffer-free and high concentration systems, improve product stability and patient tolerability while complying with regulatory standards; and (2) intellectual property challenges, including patent thickets, strongly influence. Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC12196224/. Accessed:.
- BioSpecialized. The Case for Biosimilars. Available at: https://www.biospecialized.com/case-for-biosimilars/. Accessed:.
- ResearchGate. Challenges in Inhaled Product Development and Opportunities for Open Innovation. Available at: https://www.researchgate.net/publication/49670841_Challenges_in_inhaled_product_development_and_opportunities_for_open_innovation. Accessed:.
- PatentPC. Patent Challenges Eye Medication Unraveled. Available at: https://patentpc.com/blog/patent-challenges-eye-medication-unraveled. Accessed:.
- American Pharmaceutical Review. Quality by Design (QbD) Approach to Generic Transdermal or Topical Product Development. Available at: https://www.americanpharmaceuticalreview.com/Featured-Articles/172883-Quality-by-Design-QbD-Approach-to-Generic-Transdermal-or-Topical-Product-Development/. Accessed:.
- PMC. QbD elements include the following: (1) a quality target product profile (QTPP) that identifies the critical quality attributes (CQAs) of the drug product. Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC4070262/. Accessed:.
- FDA. How Modeling Was Used to Support FDA Approval of a Topical Generic Drug Product. Available at: https://www.fda.gov/drugs/regulatory-science-action/how-modeling-was-used-support-fda-approval-topical-generic-drug-product. Accessed:.
- Drug-Dev. Nanotechnology Market: Nanotechnology Markets in Healthcare & Medicine. Available at: https://drug-dev.com/nanotechnology-market-nanotechnology-markets-in-healthcare-medicine/. Accessed:.
- BioSpace. Nanotechnology Drug Delivery Market Size to Reach USD 228.82 Billion by 2032. Available at: https://www.biospace.com/press-releases/nanotechnology-drug-delivery-market-size-to-reach-usd-228-82-billion-by-2032-on-back-of-personalized-healthcare-advancements. Accessed:.
- ResearchGate. A Case Study on Decentralized Manufacturing of 3D Printed Medicines. Available at: https://www.researchgate.net/publication/371409379_A_case_study_on_decentralized_manufacturing_of_3D_printed_medicines. Accessed:.
- MDPI. Three-dimensional printing technology is transforming pharmaceutical manufacturing. Available at: https://www.mdpi.com/1999-4923/17/3/390. Accessed:.
- FDA. Office of Generic Drugs 2022 Annual Report. Available at: https://www.fda.gov/drugs/generic-drugs/office-generic-drugs-2022-annual-report. Accessed:.
- PMC. Recently, extensive growth in bibliometric analysis has been observed. Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC11930681/. Accessed:.
- PMC. Application for a modified release formulation of a drug that is authorised in a formulation with a different release rate. Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC11209137/. Accessed:.
- PMC. Modified release products are complex dosage forms designed to release drug in a controlled manner. Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC2895434/. Accessed:.
- PMC. The dawning of the controlled release drug delivery systems began with the introduction of the Spansule® 12-hour release technology. Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC8840987/. Accessed:.
- Diamond Light Source. Understanding the journey of the active pharmaceutical ingredients. Available at: https://www.diamond.ac.uk/industry/Case-Studies/Drug-Development-Case-Studies.html. Accessed:.
- IQVIA. Sticking Point: Developing and Retaining Value with Parenteral Products. Available at: https://www.iqvia.com/-/media/iqvia/pdfs/library/white-papers/sticking-point-developing-and-retaining-value-with-parenteral-products.pdf. Accessed:.
- Sigma-Aldrich. Overcoming Challenges in Ophthalmic Formulations. Available at: https://www.sigmaaldrich.com/US/en/technical-documents/technical-article/pharmaceutical-and-biopharmaceutical-manufacturing/liquid-formulation-strategies/overcoming-challenges-in-ophthalmic-formulations. Accessed:.
- DrugPatentWatch. Innovations in Generic Drug Manufacturing and Formulation. Available at: https://www.drugpatentwatch.com/blog/innovations-in-generic-drug-manufacturing-and-formulation-transforming-products-and-reducing-costs/. Accessed:.
- PMC. Based on past experience of accessing medicines for life-threatening diseases there is a justifiable fear that access to any vaccines and treatments that are eventually developed may be hindered by patents. Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC7592140/. Accessed:.
- RAPS. Expanding Global Access to Complex Generics. Available at: https://www.raps.org/news-and-articles/news-articles/2025/2/expanding-global-access-to-complex-generics-the-ca. Accessed:.
- Congress. One of the great successes in healthcare in the past 30 years has been the introduction and widespread use of generic drugs. Available at: https://www.congress.gov/event/113th-congress/house-event/LC39280/text. Accessed:.
- FDA. Remarks by FDA Officials: Generic Drug Science Day. Available at: https://www.fda.gov/news-events/speeches-fda-officials/remarks-fda-employees-generic-drug-science-day-11282017. Accessed:.
- FDA. FDA’s Efforts to Achieve Global Regulatory Harmonization in Generic Drug Programs. Available at: https://www.fda.gov/international-programs/global-perspective/fdas-efforts-achieve-global-regulatory-harmonization-generic-drug-programs. Accessed:.
- GoodRx. Recent FDA Approval Statistics. Available at: https://www.goodrx.com/drugs/news/recent-fda-approval-statistics. Accessed:.
- FDA. Generic Drugs Program Monthly and Quarterly Activities Report. Available at: https://www.fda.gov/industry/generic-drug-user-fee-amendments/generic-drugs-program-monthly-and-quarterly-activities-report. Accessed:.
- Aprecia. FDA Approves The First 3D Printed Drug Product. Available at: https://aprecia.com/resources/press/fda-approves-the-first-3d-printed-drug-product/. Accessed:.
- Aprecia. First FDA-Approved Medicine Manufactured Using 3D Printing Technology Now Available. Available at: https://aprecia.com/resources/press/first-fda-approved-medicine-manufactured-using-3d-printing-technology-now-available/. Accessed:.
- DrugPatentWatch. Uncovering Lucrative Low-Competition Generic Drug Opportunities. Available at: https://www.drugpatentwatch.com/blog/uncovering-lucrative-low-competition-generic-drug-opportunities/. Accessed:.
- Drug-Dev. Modified Release: Alternative Strategies for Development of Modified Release Dosage Forms. Available at: https://drug-dev.com/modified-release-alternative-strategies-for-development-of-modified-release-dosage-forms/. Accessed:.
- PMC. For both brand and generic drugs, the achievement of consistent quality and therapeutic performance using drug delivery systems. Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC4627459/. Accessed:.
- PharmaSky. Complex Generic Development. Available at: https://www.pharmsky.com.au/complex-generic-development/. Accessed:.
- JAPSONline. In recent years, complex generics a special class has excel the scope of Generic Drug User Fee Act (GDUFA). Available at: https://japsonline.com/admin/php/uploads/4400_pdf.pdf. Accessed:.
- USP. What are complex generics? Available at: https://www.usp.org/sites/default/files/usp/document/ea83b_complex-generics_wp_2023-07_v3.pdf. Accessed:.
- Touro Scholar. One particular factor which, once discovered, turned into a main focus in pharmacological research. Available at: https://touroscholar.touro.edu/cgi/viewcontent.cgi?article=1146&context=sjlcas. Accessed:.
- PMC. Forty-two MR-AED formulations were studied in 3,175 healthy participants without epilepsy in 97 BE studies. Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC5340552/. Accessed:.
- PMC. In new drug development, MIDD approaches are often applied to inform clinical trial design. Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC9097888/. Accessed:.
- PMC. The pharmaceutical industry has experienced rapid growth in the area of complex injectable products. Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC9376055/. Accessed:.
- PMC. Despite being the most widely prescribed formulation, oral formulations possess several limitations. Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC11870889/. Accessed:.
- PMC. Through 2017, nine (53%) drugs approved in 2002 had been connected to 21 new formulations. Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC10398232/. Accessed:.
- PMC. A total of 15 of 27 originator drugs maintained over 70% market share eight quarters after the first generic entry. Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC12209137/. Accessed:.
- GAO. Generic drugs cost less than their brand-name, or “innovator,” counterparts. Available at: https://www.gao.gov/assets/gao-12-371r.pdf. Accessed:.
- FDA. How Generic Competition Advances Public Health by Improving Access to Affordable Medication. Available at: https://www.fda.gov/about-fda/center-drug-evaluation-and-research-cder/generic-competition-and-drug-prices. Accessed:.
- FTC. Estimating the Effect of Entry on Generic Drug Prices Using Hatch-Waxman Exclusivity. Available at: https://www.ftc.gov/sites/default/files/documents/reports/estimating-effect-entry-generic-drug-prices-using-hatch-waxman-exclusivity/wp317.pdf. Accessed:.
- Bohrium. Effect of Competition on Generic Drug Prices. Available at: https://www.bohrium.com/paper-details/effect-of-competition-on-generic-drug-prices/813145379066347520-7882. Accessed:.
- Drug-Dev. Modified Release: Alternative Strategies for Development of Modified Release Dosage Forms. Available at: https://drug-dev.com/modified-release-alternative-strategies-for-development-of-modified-release-dosage-forms/. Accessed:.
- ResearchGate. Modified Release Formulations to Achieve the Quality Target Product Profile (QTPP). Available at: https://www.researchgate.net/publication/265946962_MODIFIED_RELEASE_FORMULATIONS_TO_ACHIEVE_THE_QUALITY_TARGET_PRODUCT_PROFILE_QTPP. Accessed:.
- East Street Pharmacy. The Economic Impact of Generic Drugs on the Healthcare System. Available at: https://eaststreetpharmacy.com/the-economic-impact-of-generic-drugs-on-the-healthcare-system.html. Accessed:.
- ASPE. In this update to previous ASPE analyses, we find that in recent years new generic markets continue to be competitive. Available at: https://aspe.hhs.gov/sites/default/files/documents/510e964dc7b7f00763a7f8a1dbc5ae7b/aspe-ib-generic-drugs-competition.pdf. Accessed:.
- PubMed. Promoting substitution of lower priced generics for brand drugs once the market exclusivity period for the latter expires is a key component of the US strategy. Available at: https://pubmed.ncbi.nlm.nih.gov/34904207/. Accessed:.
- DrugPatentWatch. Generic Drug Entry Timeline: Predicting Market Dynamics After Patent Loss. Available at: https://www.drugpatentwatch.com/blog/generic-drug-entry-timeline-predicting-market-dynamics-after-patent-loss/. Accessed:.
- Fierce Pharma. Novartis CEO predicts generic industry fallout as quality takes center stage. Available at: https://www.fiercepharma.com/drug-safety/novartis-ceo-predicts-generic-industry-fallout-as-quality-takes-center-stage. Accessed:.
- Contract Pharma. The Generic Industry Faces External Challenges. Available at: https://www.contractpharma.com/exclusives/regulatory-affairs-the-generic-industry-faces-external-challenges/. Accessed:.
- Aprecia. FDA Approves The First 3d Printed Drug Product. Available at: https://aprecia.com/resources/press/fda-approves-the-first-3d-printed-drug-product/. Accessed:.
- Aprecia. First FDA-Approved Medicine Manufactured Using 3d Printing Technology Now Available. Available at: https://aprecia.com/resources/press/first-fda-approved-medicine-manufactured-using-3d-printing-technology-now-available/. Accessed:.
- ASPE. The generic injectable drug market has recently experienced numerous shortages. Available at: https://aspe.hhs.gov/sites/default/files/documents/00a4118b9da67a5e37a1e30135d17af0/aspe-generic-injectable-roi.pdf. Accessed:.
- ASPE. We discuss the effects of brand drug product hopping in more detail in Section 7.1.2. Available at: https://aspe.hhs.gov/sites/default/files/documents/20e14b66420440b9e726c61d281cc5a5/cost-of-generic-drugs-erg.pdf. Accessed:.
- PMC. The current success rate of a drug candidate, from the beginning of the clinical trial to receiving marketing approval, is about 10%–20%. Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC8212735/. Accessed:.
- FDA. Office of Generic Drugs 2021 Annual Report. Available at: https://www.fda.gov/drugs/generic-drugs/office-generic-drugs-2021-annual-report. Accessed:.
- Seed NIH. This case study breaks down the process described in our Regulatory Knowledge Guides. Available at: https://seed.nih.gov/sites/default/files/2024-01/Drug-Regulatory-Case-Study_1.pdf. Accessed:.
- Altasciences. Ophthalmic medications have a particular set of challenges that can impact their speedy and successful path to market. Available at: https://www.altasciences.com/sites/default/files/2022-09/The-Altascientist_issue-27_Ophthalmic_1.pdf. Accessed:.
- PMC. Furthermore, this review also introduces advanced manufacturing technologies such as 3D printing and hot-melt extrusion (HME). Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC11511072/. Accessed:.
- PMC. The imperfect match between the canonical economic competitive model and the reality of generic drug markets is what motivated our analysis. Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC8452364/. Accessed:.
- ResearchGate. Forces influencing generic drug development in the United States: A narrative review. Available at: https://www.researchgate.net/publication/308573425_Forces_influencing_generic_drug_development_in_the_United_States_A_narrative_review. Accessed:.
- PharmaExec. Innovation Requires Investment—and Sustained Investment Requires Sufficient Returns. Available at: https://www.pharmexec.com/view/innovation-requires-investment-and-sustained-investment-requires-sufficient-returns. Accessed:.
- INET Economics. Rethinking Pharmaceutical Innovation Policy. Available at: https://www.ineteconomics.org/perspectives/blog/rethinking-pharmaceutical-innovation-policy. Accessed:.
- Schaeffer USC. Schaeffer White Paper Highlights Failures in Generic Drug Market. Available at: https://schaeffer.usc.edu/research/paper-highlights-failures-in-the-generic-drug-market-that-are-costing-patients/. Accessed:.
- ResearchGate. The impact on cost-effectiveness of accounting for generic drug pricing: Four case studies. Available at: https://www.researchgate.net/publication/365110620_The_impact_on_cost-effectiveness_of_accounting_for_generic_drug_pricing_Four_case_studies. Accessed:.
- Gabi-Journal. A Case Study of AstraZeneca’s Omeprazole-Esomeprazole Chiral Switch Strategy. Available at: https://gabi-journal.net/a-case-study-of-astrazenecas-omeprazole-esomeprazole-chiral-switch-strategy.html. Accessed:.
- WJAR. The pharmacological discipline of In vivo–In Vitro Correlation (IVIVC) plays a crucial role in drug development. Available at: https://wjarr.com/sites/default/files/WJARR-2024-1197.pdf. Accessed:.
- PMC. The current success rate of a drug candidate, from the beginning of the clinical trial to receiving marketing approval, is about 10%–20%. Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC8212735/. Accessed:.
- PMC. Recently, extensive growth in bibliometric analysis has been observed. Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC11930681/. Accessed:.
- ASPE. The generic injectable drug market has recently experienced numerous shortages. Available at: https://aspe.hhs.gov/sites/default/files/documents/00a4118b9da67a5e37a1e30135d17af0/aspe-generic-injectable-roi.pdf. Accessed:.
- ASPE. We discuss the effects of brand drug product hopping in more detail in Section 7.1.2. Available at: https://aspe.hhs.gov/sites/default/files/documents/20e14b66420440b9e726c61d281cc5a5/cost-of-generic-drugs-erg.pdf. Accessed:.
- PMC. The pharmaceutical industry has experienced rapid growth in the area of complex injectable products. Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC9376055/. Accessed:.
- PMC. Despite being the most widely prescribed formulation, oral formulations possess several limitations. Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC11870889/. Accessed:.
- PMC. Through 2017, nine (53%) drugs approved in 2002 had been connected to 21 new formulations. Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC10398232/. Accessed:.
- IQVIA. Sticking Point: Developing and Retaining Value with Parenteral Products. Available at: https://www.iqvia.com/-/media/iqvia/pdfs/library/white-papers/sticking-point-developing-and-retaining-value-with-parenteral-products.pdf. Accessed:.
- PMC. The imperfect match between the canonical economic competitive model and the reality of generic drug markets is what motivated our analysis. Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC8452364/. Accessed:.
- ResearchGate. Forces influencing generic drug development in the United States: A narrative review. Available at: https://www.researchgate.net/publication/308573425_Forces_influencing_generic_drug_development_in_the_United_States_A_narrative_review. Accessed:.
- PharmaExec. Innovation Requires Investment—and Sustained Investment Requires Sufficient Returns. Available at: https://www.pharmexec.com/view/innovation-requires-investment-and-sustained-investment-requires-sufficient-returns. Accessed:.
- INET Economics. Rethinking Pharmaceutical Innovation Policy. Available at: https://www.ineteconomics.org/perspectives/blog/rethinking-pharmaceutical-innovation-policy. Accessed:.
- Schaeffer USC. Schaeffer White Paper Highlights Failures in Generic Drug Market. Available at: https://schaeffer.usc.edu/research/paper-highlights-failures-in-the-generic-drug-market-that-are-costing-patients/. Accessed:.
- ResearchGate. The impact on cost-effectiveness of accounting for generic drug pricing: Four case studies. Available at: https://www.researchgate.net/publication/365110620_The_impact_on_cost-effectiveness_of_accounting_for_generic_drug_pricing_Four_case_studies. Accessed:.
- Gabi-Journal. A Case Study of AstraZeneca’s Omeprazole-Esomeprazole Chiral Switch Strategy. Available at: https://gabi-journal.net/a-case-study-of-astrazenecas-omeprazole-esomeprazole-chiral-switch-strategy.html. Accessed:.
- WJAR. The pharmacological discipline of In vivo–In Vitro Correlation (IVIVC) plays a crucial role in drug development. Available at: https://wjarr.com/sites/default/files/WJARR-2024-1197.pdf. Accessed:.
- PMC. The current success rate of a drug candidate, from the beginning of the clinical trial to receiving marketing approval, is about 10%–20%. Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC8212735/. Accessed:.
- PMC. Recently, extensive growth in bibliometric analysis has been observed. Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC11930681/. Accessed:.
- ASPE. The generic injectable drug market has recently experienced numerous shortages. Available at: https://aspe.hhs.gov/sites/default/files/documents/00a4118b9da67a5e37a1e30135d17af0/aspe-generic-injectable-roi.pdf. Accessed:.
- ASPE. We discuss the effects of brand drug product hopping in more detail in Section 7.1.2. Available at: https://aspe.hhs.gov/sites/default/files/documents/20e14b66


























