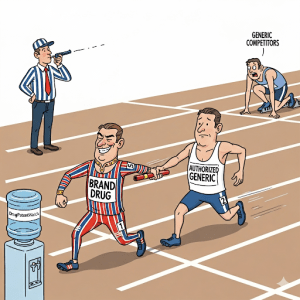
The rise of authorized generics has become a defining feature of U.S. pharmaceutical markets in the past decade. In plain terms, an authorized generic is an exact copy of a brand-name drug sold under a generic label. Unlike independent generics developed by competitors, authorized generics are simply the brand company (or its licensee) marketing its own approved drug without the brand name on the label. As the FDA explains, “‘Authorized generic’ […] is an approved brand name drug that is marketed without the brand name on its label… Other than the fact that it does not have the brand name on its label, it is the exact same drug product as the branded product”. In other words, the product is identical at the molecular level – only the label changes. This practice blurs the line between brand and generic, introducing a strategic twist for companies looking to defend market share as patents expire.
In this article we dive deep into the prevalence and timing of authorized generics in the U.S. pharmaceutical market over the last 10 years. We’ll examine how often brand companies launch these copies, when they choose to launch them (especially relative to patent and exclusivity events), and the impacts on competition and pricing. Throughout, we will draw on the latest industry data and expert analysis (including insights from DrugPatentWatch and the FDA) to illuminate trends that matter to pharmaceutical executives. We’ll also glance at how other markets handle analogous situations. By the end, you’ll have a clear, data-driven view of how authorized generics fit into market strategies, and how timing plays a critical role in their effectiveness.
“An authorized generic… is an approved brand name drug that is marketed without the brand name on its label… It is the exact same drug product as the branded product.” – U.S. Food & Drug Administration
Background: The Role of Authorized Generics
Authorized generics emerged as a strategy under the Hatch-Waxman framework of the 1980s. That law created a balance: it gave the first generic entrant up to 180 days of marketing exclusivity, while also enabling brand companies to protect revenue. Under Hatch-Waxman, a brand-name drug that is about to face generic competition can legally market the same product under a generic label. Because an authorized generic is simply sold under the brand’s original New Drug Application (NDA), it does not need a separate approval – the FDA only requires the brand to notify them of marketing. This means the brand can drop its own generic version onto the market at patent expiry or even beforehand, competing directly with independents.
The effect? Authorized generics effectively place another low-priced pill into the market slot intended for independent generics. This can soothe short-term public pressure for lower prices (by “doubling” the competition), but it also dilutes the advantage that the first-filer generic would otherwise enjoy. As noted by one executive, the plan is often “to maximize profit at this point,” even if it means sacrificing some price erosion. In short, authorized generics let brands “compete with themselves,” as if they have an extra generic arm in the fight. Understanding how frequently and when brands deploy this tactic is key to predicting market outcomes.
Regulatory Framework and Timing: Hatch-Waxman and Exclusivity
A core factor in authorized generic timing is the 180-day exclusivity awarded to the first approved generic firm under Hatch-Waxman. This special exclusivity means that for six months, only the first filer’s generic (plus any authorized generic) can enter the market. A brand-name firm can legally launch an authorized generic during that six-month window. In practice, brands have learned to leverage this period.
As the FTC has documented, it’s “increasingly common for brand-name drug makers to start marketing authorized generics at the same time a generic firm is beginning its 180-day marketing exclusivity period”. During that period, consumer prices tend to be lower than they would have been without an authorized generic. The FTC’s research found that retail prices in the 180-day window are typically 4–8% lower when an authorized generic is present, and wholesale prices are 7–14% lower. This confirms that even a single authorized generic can moderate prices. But there’s a trade-off: the first-filer generic’s revenues drop sharply – 40–52% lower during exclusivity. In other words, the brand’s copy steals almost half of the generic’s sales in that period.
The effect beyond the exclusivity window is also significant. FTC analysis showed first-generic revenues continue to lag (53–62% lower over 30 months post-exclusivity) if they face an authorized generic. In fact, by raising the stakes for first filers, brands may encourage generic challengers to delay entry. The FTC warned of collusive settlements where a brand promises not to launch an authorized generic if the generic delays its launch, effectively extending the brand’s monopoly. Thus the timing of authorized generics – especially around the exclusivity window – has been shaped by both strategy and regulation.
Internationally, the environment differs. For example, in the EU generics regulatory system, a brand cannot simply re-label its drug immediately after patent expiry. The European Medicines Agency (EMA) requires that generics can only be authorized after at least 10 years of data exclusivity have passed. That effectively means the brand loses its monopoly on any identical product before generics can legally enter. As a result, “authorized generics” in the strict U.S. sense are not an available strategy in Europe. Instead, brand companies in Europe might license their drugs to generic manufacturers or sell “branded generics,” but these must wait until exclusivity periods end. In short, the U.S. market’s early-launch environment is relatively unique.
Authorized Generic Launch Trends in the U.S. (2010–2023)
Over the last decade-plus, authorized generics have become a fixture of U.S. pharmaceutical markets. A recent comprehensive study (Health Affairs, 2023) found 854 authorized generic launches from 2010 through 2019. That works out to roughly 85 launches per year on average. Notably, launch activity peaked around 2014 and has varied since. This study linked FDA and sales data to profile these launches and uncovered interesting patterns.
First, timing patterns: roughly three-fourths of authorized generics in markets where independent generics were launching came after the generic launch. In other words, it’s common for brands to wait until a competitor’s generic is already on the shelf. However, when one focuses on markets where a first generic was eligible for 180-day exclusivity, about 70% of authorized generics launched before or during that exclusivity period. This indicates that brands often aim to hit the market in tandem with the first-filer generic. By doing so, they maximize their share of the limited-market window. DrugPatentWatch notes that these launches are “strategic,” often occurring when a brand’s revenues are highest or to coincide with a new rival entering.
To translate this: imagine a brand holder whose blockbuster is going off-patent. If an independent generic is poised to enter with six months’ exclusivity, the brand often times its own copy to appear simultaneously. That authorized generic then races head-to-head with the new generic. According to one analysis, in such cases the brand’s product and the new generic each capture substantial market share, often leaving the brand itself smaller in terms of prescriptions. This high-turnover window is the sweet spot for authorized generics. If they come too late, after competition is fully established, their impact is smaller. If they come too early (before a generic even exists), they may simply cannibalize brand sales without a rival to share demand.
Second, vertical trends: the number of authorized generic launches has grown overall, and the FDA now tracks them explicitly. By 2019 there were “nearly 1,200 authorized generics approved in the U.S.” according to the FDA. That total continues to rise as more drugs face off-patent dates. For context, the same year FDA statistics showed roughly 20,000 generic approvals overall. So about 5–6% of those were authorized generics by count. Another way to look at it: cutting-edge analyses found that nearly half of drugs seeing new generics from 2012–2017 also saw an authorized generic launched at the same time. In practice this means that if a blockbusting pill goes generic, there’s a significant chance the brand will roll out its own unbranded twin.
Therapeutic and Formulation Patterns: Authorized generics span diverse drug classes. Some are little-genericized sectors like certain inhalers (e.g. albuterol inhalers had high authorized generic use) and ADHD drugs (methylphenidate, amphetamine). Others are widely-used specialty pills: an illustrative example from Medicaid data was levothyroxine (a thyroid drug) where the authorized generic had a substantial share of usage. In fact, out of all authorized generics identified in Medicaid from 2014–2020, levothyroxine’s AG captured about 19% of market prescriptions, and albuterol AGs hit 32% (driven by two separate AG launches). A general takeaway is that authorized generics are not limited to obscure drugs; they often accompany mid- to large-market brands with high volume.
Authorized Generics in the Medicaid Market (2014–2020)
Looking at Medicaid (which uses volume data from state drug rebate reports) provides a real-world gauge of authorized generics’ market presence. From 2014 to 2020, Medicaid patients filled roughly 4.6 billion prescriptions across branded, generic, and authorized versions. Of these, about 175 million (4%) were for authorized generics. Independent (traditional) generics accounted for 80% of all fills, and branded drugs 11%. In other words, AGs are a small slice of total volume. However, that understates their punch in the slots where they exist.
Among drugs that had an authorized generic available at any point, AGs grabbed roughly 16% of prescriptions on average when active, versus 75% for independent generics. This means that when an AG is on the table, it takes about one-sixth of the market for that molecule/formulation. Notably, AGs’ share peaked in 2014 around 20% then declined to 11% by 2020 (if one excludes a couple of outliers like albuterol inhalers introduced in 2019). In specific cases, an AG can dominate: for albuterol inhalers (2019 onward), one AG variant reached 51% of use. This underscores that AGs can sometimes overshadow even established generics.
These Medicaid data also reveal the prevalence of authorized generics: 2014–2020 saw 1023 distinct AG formulations, covering 528 different NDAs. This matches closely with the FDA’s own reported list (1041 AGs by end of 2020). In short, about one in five of the drugs benefiting from first-time generics in this period had an AG component. For executives, this means that for many products, strategy around generics must consider an AG playbook.
Strategic Motivations for Authorized Generics
Why do brand firms launch authorized generics? The motivations are strategic and rooted in market economics. Here are some key reasons:
- Defend Revenues: At patent expiry, sales usually plummet as true generics undercut price. By adding an authorized generic to the mix, the brand company recaptures some volume that would otherwise go exclusively to competitors. PDL BioPharma’s CEO, Dominique Monnet, put it plainly about the blood pressure drug Tekturna: they wanted “to maximize profit at this point… [so] the economics would still be very favorable to us” even if prescriptions dropped. In short, they weren’t aiming to protect margins (the AG often sells at similar or slightly lower price) but to protect sales volume.
- Leverage Distribution: Some U.S. payers and pharmacies automatically substitute generics for brands (“generic substitution laws”). An authorized generic, being identical to the brand, slips into this system effortlessly. As one analyst noted, if a drug is on the formulary, “even if the authorized generic isn’t much cheaper than the brand, it’s almost like a no-brainer to roll one out”. This ease of distribution – and the lack of need to educate providers about a new molecule – is a big appeal for brands.
- Strategic Negotiation: Brands can use the prospect of launching an AG as a bargaining chip in patent settlements. By threatening to erode a generic competitor’s 180-day profits, the brand can extract concessions. The FTC highlighted deals where brands promise not to bring an AG in exchange for the generic delaying its launch. In this way, AGs become part of settlement strategy, sometimes to consumers’ detriment.
- Policy Gaming: More recently, companies have used AGs to navigate payment systems. A 2024 example is GSK’s handling of Flovent. Facing new Medicaid rebate penalties under the Inflation Reduction Act, GSK withdrew Flovent and put an identical authorized generic in its place. Because the AG was technically a “new product,” the punitive rebates did not apply. (Congress and PBMs pushed back on this maneuver, but it illustrates how sophisticated brands have become.)
- Competing with Itself vs. Patents: When patents for a drug with thin patent defenses or limited appeal come off-market, launching an AG is often simpler than litigating new patents or seeking incremental exclusivities. In effect, the brand “dilutes” the generic opportunity through its own label, making the first-filer generic earn a much smaller return. According to research firm Cutting Edge Information, authorized generics yield “by far the most profitable” returns of any strategy, “returning $50 for every dollar invested”, far more than any typical market introduction.
These factors show that authorized generics are not random occurrences but deliberate strategic moves. For executives, understanding whether a competitor or your own company is likely to use an AG is akin to mapping out the chessboard for a patent expiry.
Case Examples: Brand Strategies in Action
Consider some real-world illustrations of how authorized generics play out:
- Tekturna (aliskiren): This ACE inhibitor became a textbook case. In 2018, when independent generics were set to launch, PDL BioPharma (which owned Tekturna) launched its own authorized generic. PDL’s CEO announced that this gave PDL “a distinctive competitive advantage”. Indeed, PDL even disbanded the Tekturna sales force, confident the AG would carry the load. The authorized generic, priced only modestly below the brand, captured substantial volume at launch. PDL later admitted that it valued the side-by-side launch as more profitable even with rebates factored in.
- EpiPen (epinephrine autoinjector): After Mylan hiked EpiPen prices by 400% in 2016, public fury led the company to introduce an authorized generic in 2017. While Mylan’s motivation was to quell outrage rather than face a generic challenge, the launch underscores how AGs can be used for public relations and market defense. (Mylan’s AG had identical dosing to EpiPen and was effectively the same product.) It arrived quickly, essentially softening the PR hit of the price increase.
- Humalog (insulin lispro): In 2023, Eli Lilly offered a generic insulin lispro, but it was technically an authorized generic of Humalog. The list price was half of Humalog’s list price, earning cheers about lowering insulin costs. Behind the scenes, experts note Lilly’s rebates meant the net price of Humalog was already near that point, so Lilly was “not taking any money out of the system”. Critics call this a “parlor trick,” since the authorized generic avoids the brand’s rebate mechanism. Yet it did allow Lilly to market “lower-cost insulin” for uninsured patients under political pressure.
- Analgesics and other classes: Authorized generics have appeared across many classes, including statins (e.g., atorvastatin), ADHD medications (e.g., Adderall XR), antibiotics (e.g., azithromycin), and more. Often a company will wait until the moment of first generic entry and then roll out an authorized version to slice into the generic’s demand. Each market has its own story, but the pattern repeats: the brand can quickly convert some of its prescription volume to an unbranded label.
These stories illustrate that authorized generics are used for different purposes – profit-maximization, PR, or strategic brinkmanship – depending on the product context. For executives, each patent cliff is both a threat and an opportunity: the calculus of launching an AG versus cutting price versus litigation is complex, but it’s become a routine part of life after patent.
Market Impact: Prices and Competition
How do authorized generics affect prices and competition? The short answer: they drive down prices modestly in the near-term, but complicate long-term competitive dynamics.
As noted, FTC studies consistently show that an authorized generic present during the 180-day window does yield lower prices than if the generic were alone. For example, one analysis found retail prices 4.2% lower on average with an AG in the 180-day period, and wholesale prices 6.5% lower. Over longer horizons, the FTC final report noted 4–8% lower retail prices and 7–14% lower wholesale prices with an AG during exclusivity. These are statistically significant, if not earth-shattering, reductions. In practice, authorized generics add another low-cost option for payers and patients, but usually only as cheap as (or a bit cheaper than) the brand’s generic.
Thus, the immediate benefit to patients can be a few percent savings. Some brand proponents (like PhRMA) highlight these savings, arguing that AGs “increase competition…and result in significant cost savings”. Indeed, if one independent generic were to hold a monopoly, prices could temporarily be higher – adding an AG adds competition.
However, these short-term savings come with a catch. Because AGs blunt the exclusive profits of the first-filer generic, they can weaken the incentive for generic entrants. Many experts note that AGs essentially “stave off” independent competition in the long run. With an AG already claiming 30–50% of market share during exclusivity, a new generic entrant might find the return on litigation and development too low. This can lead to fewer generics being launched at all. One industry professor summarized it: “That’s the game. And drug companies have become masters at this.” The concern is that over time, consumers might face fewer choices or higher prices overall because AGs reduce the financial lure of first mover generics.
Empirical evidence on this longer-term effect is still limited, as noted by many analysts. But the narrative is clear: authorized generics help brands smooth the descent of their sales and cut prices modestly, at the cost of undercutting generic incentives. Pharmaceutical executives need to weigh both sides: is the extra revenue to the brand worth potentially a slower pace of true generic competition? Each situation may differ, but it remains a key policy and business question.
Authorized Generics vs. Independent Generics: Market Share Dynamics
It’s instructive to compare market share between authorized generics (AGs) and independent generics (IGs). By definition, an AG only exists alongside at least one IG (the first filers, and eventually multi-source generics). Data suggests that in aggregate, independent generics still dominate.
For example, in Medicaid usage during 2014–2020, AGs accounted for 16% of prescriptions in products where an AG was available, while independent generics took 75%. Even if we note that this was a decline from 20% AG share in 2014 down to 11% by 2020, it shows that IGs claim roughly three-fourths of the market volume on average. In practice, think of it as: when all generics are on the shelf, about 80% of volume goes to traditional generics and only ~20% to the brand’s version – though that brand share is itself low relative to generics. (Brand-name versions took ~6% in that mix.)
In another view, for 227 drugs that faced first-time generic entry in 2012–2017, 45% had a simultaneous AG introduction. In those cases, the AG on average took 30% of prescriptions in the first 3 years. Thus, of the initial generic market, about one-third is attributable to the brand’s authorized copy.
However, some drugs are outliers. As we saw, certain high-use drugs like albuterol inhalers have AGs that flipped majority usage (51%). On the other hand, many AGs capture only single-digit shares. For instance, when atorvastatin (Lipitor) went multi-source, the atorvastatin AG had only about 10% of prescriptions (with the rest mostly generics and a small brand chunk). This variance depends on pricing, formulary dynamics, and patient acceptance. Sometimes an AG is priced only marginally below brand, making it attractive to payers. Other times, if the brand heavily discounts via rebates, a newly listed AG might seem expensive and gain little traction, as happened with Flovent’s AG attempt.
Overall, while authorized generics can capture a significant slice of the market, independent generics remain the main competitors in volume. Brand executives often see an AG as a supplement – recouping maybe 20–40% of what generics would have taken – but they rarely expect to double their volume back to brand-era levels.
Ex-US Perspective: Branded Generics and Global Context
It’s worth noting that the authorized generic phenomenon is largely a U.S. artifact. In many other markets, brand manufacturers have different ways to maintain market share. For instance, in Europe, companies might launch “branded generics” – essentially generics produced by the originator’s affiliate or licensee, but often still sold under a different brand name. In India and other emerging markets, companies frequently introduce “branded generics” upon patent expiry, sometimes at substantial discounts. These serve a similar purpose but are treated as independent generics in those markets.
Importantly, regulatory timelines differ. As noted, Europe’s data and market exclusivity means a brand usually cannot get an AG onto the market until at least 10 years after first approval, and then the brand itself would typically just continue selling the reference product. There is no separate AG designation in most countries. Instead, Europe sees multiple independent companies (including originators’ generics divisions) competing later on. Generic penetration in Europe varies widely (from 20% by volume in some countries to 80% in others), but the strategies brands use to adapt often involve portfolio management rather than U.S.-style AG launches.
In emerging markets like China and India, brand firms often license local companies to produce identical copies. For example, many big pharma firms have authorized generic partnerships in India where the generic has its own name but is made by the innovator’s local arm. This blurs lines but differs in that the regulatory path is the standard generic route. Comparing globally, the unique U.S. mix of quick generic entry and 180-day exclusivity means American brands have honed the AG tactic more than anyone else.
For our purposes, the key takeaway is that the strategic logic of protecting revenue around patent expiry is universal, but how it plays out depends on local rules. U.S. executives should remain aware of these differences when operating internationally. For instance, FDA’s 1,200 approved AGs is not mirrored by EMA, but in both regions, brand companies seek first-mover advantages in their own way.
Policy and Legal Considerations
Authorized generics have also drawn policy scrutiny. Legislators and regulators wonder whether this practice helps or hinders the goals of generic competition. On one hand, AGs increase patient access to lower prices sooner. On the other, they can reduce the incentive for generics to challenge patents, potentially slowing overall generic penetration.
Regulatory bodies track and publish AG data. The FDA, for example, maintains a quarterly list of authorized generics as required by law. The FTC has issued multiple reports on the topic, emphasizing both consumer price effects and competitive impacts. In policy debates, industry lobbies (like PhRMA) argue that AGs “reduce prices and result in significant cost savings”. Critics counter that AGs are “not generic drugs” at heart (they do nothing to truly expand competition) and complain that deals involving AGs can forestall real generic entry.
For executives, the evolving legal context matters too. For example, antitrust authorities have examined patent settlements where AG rights are part of the terms. The Supreme Court has outlawed “pay-for-delay” involving cash but companies have shifted to compensation schemes around AGs. Managers should keep an eye on FTC guidance, new Congressional inquiries, and even state legislatures (some of which consider AG-related legislation in drug pricing laws).
An interesting recent twist is the use of AGs in non-patent settings, such as the Medicaid rebate change mentioned earlier. The moral: creative use of AGs can touch policy levers beyond patents, so compliance teams must be vigilant.
Implications for Pharmaceutical Executives
What does all this mean for you, the pharmaceutical executive? Several points stand out:
- Strategic Planning: For each key product approaching patent expiry, plan for all scenarios: brand-only, independent generics, and authorized generics. Model the financial trade-offs, knowing that AGs can take roughly one-third of the market volume that an independent generic would otherwise enjoy.
- Timing is Everything: If you consider launching an AG, timing it correctly can amplify benefits. The data show that most high-impact AG launches coincide with first-generic entry. Decide whether to beat the first filer in the market, meet it, or stay back. Each approach has different implications for revenue, pricing, and relations with potential generic partners.
- Pricing Strategy: Remember that simply undercutting your own brand price may not be the goal. Authorized generics often sell at a modest discount to brand price, capturing volume rather than maximizing per-unit margin. Also consider rebate structures: as seen with Lilly’s Humalog, skipping rebates (as an AG typically does) can paradoxically make the AG net more expensive to certain payers. Be careful: list price vs net price dynamics can nullify expected savings.
- Communications and Policy: Be proactive in communicating with payers and the public. As seen with EpiPen, an AG can ease consumer outrage. However, aggressive use of AGs can trigger legislative pushback. Monitor legal developments (FTC, state laws, healthcare bills) that might restrict AG agreements or redefine their taxonomy.
- Competition Analysis: Keep a close watch on competitors’ pipelines. If another company’s generic is nearing approval, consider what your authorized generic policy will be. Conversely, if you are a generic company, anticipate AG moves by your rivals; factor in those share-stealing effects when valuing your exclusivity and negotiating settlements.
- Global Lens: If operating in multiple markets, adapt your playbook. Countries without U.S.-style AG pathways require different tactics. Branded generics or licensing deals might fill similar roles abroad. Study local rules: for example, in the EU, market exclusivity and intellectual property rules will dictate when and how a brand might preserve revenue post-patent.
Ultimately, the data show AGs are common (roughly one launch per week in 2018) and strategically timed. As one pharmacy executive bluntly put it, they’re “just another tactic for drug manufacturers to improve profitability.” That makes knowledge of this tactic crucial for competitive decision-making. Armed with the numbers above – from 4% share in Medicaid all the way to 854 launches in a decade – leaders can better predict market behavior and seize control of their product’s lifecycle.
Key Takeaways
- Definition & Strategy: Authorized generics are brand-company versions of their own drugs sold under a generic label. They let brands share the generic market while preserving sales.
- Market Prevalence: Over 2010–2019, 854 authorized generics launched, peaking around 2014. By 2019, ~1200 authorized generics were FDA-approved.
- Timing Patterns: About 70% of authorized generics hit the market before or during the first-generic’s 180-day exclusivity. Brands often synchronize AG launch with first-generic entry to capture peak revenues.
- Market Impact: Authorized generics claim roughly 30–40% of market share during exclusivity, significantly cutting the first-generic’s revenues. Short-term, they lower prices by ~4–8% in the exclusivity window, but they erode independent generic incentives.
- Long-Term Effects: While adding short-term supply and price competition, authorized generics can suppress entry incentives. Critics note they “stave off generic competition” and reduce the number of true generics launched.
- U.S. Specific: This phenomenon is chiefly American due to unique FDA and Hatch-Waxman rules. In Europe, generics must wait 10+ years post-launch, so brands cannot insert identical copies immediately. Instead, European firms rely more on licensing or branded generics.
- Strategic Deployment: Companies deploy authorized generics to maximize profits, as PDL CEO Monnet admitted. AGs are highly profitable (up to $50 gain per $1 spent) and used to negotiate deals or dodge price controls (e.g. GSK’s 2023 Flovent maneuver).
- Business Planning: Pharma executives must factor AGs into patent-expiry planning. Monitor competitor pipelines and payers closely. Decide if launching an AG is worth the tradeoff of lower wholesale prices vs. retained volume. In R&D and legal strategy, the existence of AGs may affect decisions on further patent challenges and settlements.
- Patient Access vs. Incentives: Authorized generics can improve access briefly, but the long-run policy debate continues. Future research is needed to weigh consumer benefits against potential reductions in generic competition.
FAQs
- What exactly is an “authorized generic” and how is it different from a regular generic?
An authorized generic is the same drug as the brand-name product, marketed under the original NDA without the brand name. Unlike independent generics (which go through an ANDA pathway), authorized generics have no new approval process and are typically launched by the brand holder or its licensee. They often appear at the same time as first independent generics and are identical in formulation, meaning pharmacists and patients see no difference except the label. - How common are authorized generics in the U.S. market?
Very common. In recent years, about 1–2 new authorized generics have launched per week on average. By the end of 2019, the FDA had approved nearly 1,200 authorized generics. A study of 2010–2019 found 854 such launches. Many blockbusters coming off-patent now have an associated authorized generic, so managers should expect this in many therapy areas. - When do companies typically launch authorized generics?
Most often, at or just before the time of first generic entry. About 70% of authorized generics launch before or during the first-generic’s 180-day exclusivity window. This strategy allows brands to share the high-revenue launch window. Some AGs do come out earlier or later, but the highest-impact launches are aligned with generic competition. - Do authorized generics lower drug prices?
Generally, yes, but modestly. FTC studies show that when an AG competes with the first generic, average prices are a few percent lower than they would be without it. However, the brand’s rebates and payers’ negotiated discounts can mean the net effect on spending is complex. Importantly, AGs reduce prices mostly in the short term; long-term price declines depend more on the overall number of generics and market competition, which AGs can dampen by reducing profit incentives for others. - How should a pharma company decide whether to launch an authorized generic?
It’s a strategic choice. Key considerations include: How much revenue you’ll sacrifice by competing on price, how much market share you can reclaim, and what effect it will have on your remaining brand sales and on the generic competitor’s profits. You should model whether capturing (say) 30% of volume at a generic price yields higher or lower profit than just keeping your brand alone. Also consider legal settlements (often involving first-filers) and payer reactions. There’s no one-size-fits-all answer; decisions are usually made on a drug-by-drug basis considering market dynamics and corporate objectives.
Sources: Authoritative data and expert analyses on authorized generics were drawn from government and industry publications, including U.S. FDA definitions and lists, FTC studies, academic research, and news investigations. DrugPatentWatch (see also their blog post on this topic) and KFF Health News provided industry context and quotes. These cited sources form the basis of the analysis above.