Last updated: July 27, 2025
Introduction
Prednisolone, a synthetic corticosteroid, has long served as a cornerstone in managing inflammatory and autoimmune conditions. Its versatility, well-established efficacy, and cost-effectiveness have entrenched it as a vital drug within the pharmaceutical landscape. As global healthcare systems evolve and novel therapies emerge, understanding prednisolone’s market dynamics and financial trajectory becomes critical for stakeholders including manufacturers, investors, and healthcare policymakers. This analysis offers a comprehensive overview of the factors shaping prednisolone’s market, assessing demand drivers, competitive landscape, regulatory influences, and potential future trends.
Pharmacological Profile and Therapeutic Applications
Prednisolone exhibits potent anti-inflammatory and immunosuppressive properties, making it indispensable in treating asthma, allergies, rheumatoid arthritis, dermatological conditions, and certain hematologic disorders [1]. The drug’s oral, injectable, and topical formulations provide broad administration versatility, further cementing its widespread use. Its longstanding generic presence ensures affordability, supporting its popularity in both developed and emerging markets.
Market Demand Drivers
1. Growing Incidence of Chronic Conditions
The global rise in autoimmune diseases and inflammatory disorders propels demand for corticosteroids like prednisolone. Notably, increased prevalence of rheumatoid arthritis and asthma contributes to sustained consumption. According to the Global Burden of Disease Study, these conditions remain among the leading causes of disability worldwide, underpinning a steady need for corticosteroids.
2. Healthcare Accessibility and Affordability
Prednisolone’s status as a low-cost, off-patent medication allows broad access, particularly in low- and middle-income countries (LMICs). As healthcare infrastructure improves in these regions, prescription rates are expected to rise, further boosting demand [2].
3. Emergency and Acute Care Use
The drug’s utility extends to acute allergic reactions and exacerbations of chronic conditions. Its established role in hospital protocols sustains consistent demand, especially amidst global health crises that exacerbate inflammatory responses.
Market Dynamics
1. Patent Expiry and Generic Competition
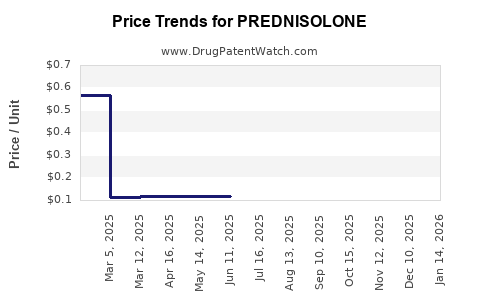
Prednisolone’s patent expiry in the early 2000s catalyzed a proliferation of generic formulations, significantly decreasing prices and expanding market reach. This commoditization enhanced accessibility but intensified price competition, compressing profit margins for manufacturers [3].
2. Manufacturing and Supply Chain Considerations
Global supply chains, predominantly based in Asia, especially India and China, produce the bulk of prednisolone supplies [4]. Recent disruptions, including geopolitical tensions and COVID-19-related logistical challenges, have occasionally led to shortages, impacting availability and pricing dynamics.
3. Regulatory and Quality Standards
Regulatory agencies enforce stringent quality standards for corticosteroids. Variations in regulatory environments, especially across emerging markets, influence manufacturing costs and market entry strategies. Conversely, quality concerns have prompted some markets to favor established suppliers or trusted APIs, impacting competitive positioning.
4. Emerging Alternatives and Therapeutic Shifts
While prednisolone remains a primary corticosteroid option, newer drugs with targeted mechanisms and fewer systemic side effects, such as biologics for autoimmune diseases, threaten its market share. However, due to cost and familiarity, prednisolone retains dominance in many settings [5].
Financial Trajectory and Market Outlook
Historical Revenue Trends
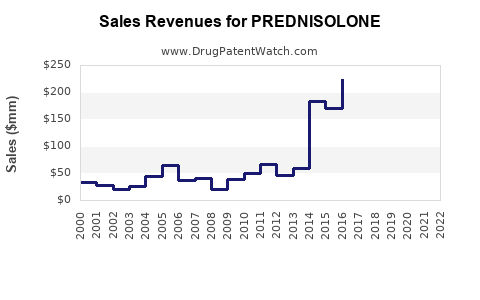
Globally, prednisolone’s annual sales have historically ranged between $500 million and $1 billion, with significant regional variations. The generic drug market’s expansion contributed to consistent revenue streams, particularly in LMICs where cost considerations dominate treatment choices [6].
Future Projections
Forecasts suggest moderate growth for prednisolone over the next five years, driven by:
- Continued demand in developing regions.
- Growing incidence of autoimmune and inflammatory conditions.
- Policy initiatives promoting affordable essential medicines.
However, growth may be tempered by:
- Increasing preferences for targeted biologics.
- Price erosion due to intensified generic competition.
- Regulatory barriers, especially concerning quality assurance.
The compound annual growth rate (CAGR) of prednisolone sales is expected to hover around 2-3% globally, with higher potential in underserved markets. Advanced formulations, such as combined therapies or controlled-release variants, may open niche segments, although their commercial impact remains limited.
Market Players and Investment Opportunities
Major generic manufacturers like Sun Pharma, Teva, and local pharmaceutical companies dominate the prednisolone market. Strategic investments focus on expanding manufacturing capacity, ensuring supply chain resilience, and adhering to quality standards to maintain competitiveness.
Regulatory and Policy Environment
Global health policies emphasize affordable corticosteroids as essential medicines, reinforcing their market stability. The World Health Organization (WHO) classifies prednisolone as an essential medicine, facilitating subsidies and inclusion in national formularies, especially in LMICs [7].
Regulatory harmonization efforts, including mutual recognition agreements, streamline approval processes and foster market access. Yet, disparities in regulatory rigor can influence market dynamics, creating barriers and opportunities.
Market Challenges
-
Side Effect Profile: Long-term use of prednisolone is associated with adverse effects—osteoporosis, hyperglycemia, adrenal suppression—prompting clinicians to consider alternatives.
-
Price Sensitivity: Budget constraints limit the use of corticosteroids in certain markets, pressuring manufacturers to maintain low prices.
-
Emerging Disease Contexts: The COVID-19 pandemic underscored corticosteroids’ role, particularly dexamethasone. While prednisolone is similar, shifts in treatment protocols could influence its utilization patterns [8].
Future Outlook
Prednisolone’s role as an affordable, effective corticosteroid ensures its continued relevance, particularly in resource-constrained settings. Nevertheless, innovation, such as developing formulations with improved safety profiles or targeted delivery systems, could extend its lifecycle. Additionally, a looming focus on personalized medicine may stimulate research into optimized corticosteroid regimens, influencing future demand.
Key Takeaways
- Prednisolone maintains steady global demand underpinned by its efficacy, affordability, and extensive generic availability.
- Market growth is moderate, primarily fueled by increased autoimmune disease prevalence and expanding healthcare access in LMICs.
- Competitive dynamics are characterized by intense price competition, supply chain considerations, and regulatory standards.
- Emerging alternatives such as biologics pose a long-term threat but currently only complement prednisolone’s role in treatment algorithms.
- Ensuring high-quality manufacturing and regulatory compliance remains critical for market sustainability.
FAQs
1. How does prednisolone compare to other corticosteroids in terms of efficacy and safety?
Prednisolone offers comparable efficacy to other corticosteroids like prednisone and dexamethasone in managing inflammation. Its safety profile is well-characterized, with long-term use associated with adverse effects typical of corticosteroids. Choice depends on disease indication, dosing, and patient factors.
2. What regional factors influence prednisolone market growth?
Developing countries exhibit higher growth potential owing to increasing disease burden and reliance on affordable treatments. Regulatory environments and healthcare infrastructure quality also impact market expansion.
3. How are supply chain disruptions affecting prednisolone availability?
Disruptions, particularly during the COVID-19 pandemic, have occasionally led to shortages. Given manufacturing primarily in Asia, geopolitical issues and logistical hurdles can impact global supply, influencing availability and pricing.
4. Will the introduction of new drug delivery systems affect prednisolone’s market?
Potentially. Novel formulations with improved safety, targeted delivery, or controlled-release properties could expand indications and improve adherence, sustaining interest in prednisolone derivatives.
5. What role do governmental policies play in prednisolone’s market?
Recognition as an essential medicine enables government subsidies and inclusion in public health programs, fostering market stability and encouraging manufacturers to allocate resources for production and quality compliance.
References
[1] Katzung, B. G., Masters, S. B., & Trevor, A. J. (2018). Basic and Clinical Pharmacology. McGraw-Hill Education.
[2] World Health Organization. (2017). WHO Model List of Essential Medicines.
[3] IMS Health. (2014). Global Trends in Generic Drug Market.
[4] Indian Pharmaceutical Alliance. (2021). API Manufacturing Landscape.
[5] Smolen, J. S., et al. (2016). "Biologic and targeted synthetic DMARDs for rheumatoid arthritis." The New England Journal of Medicine.
[6] Market Research Future. (2022). Global Corticosteroids Market Analysis.
[7] WHO. (2019). Essential Medicines in Primary Healthcare.
[8] The RECOVERY Collaborative Group. (2020). "Dexamethasone in Hospitalized Patients with Covid-19." The New England Journal of Medicine.