LIDOCAINE Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Lidocaine, and when can generic versions of Lidocaine launch?
Lidocaine is a drug marketed by Alembic, Alkem Labs Ltd, Amneal Pharms, Aurobindo Pharma Ltd, Belmora Llc, Chartwell Rx, Cosette, Encube, Fougera Pharms Inc, Geneyork Pharms, Glenmark Pharms Ltd, Macleods Pharms Ltd, Quagen, Rising, Septodont Inc, Strides Pharma, Sun Pharma Canada, Teva Pharms Usa, Vitruvias Therap, Actavis Labs Ut Inc, Amneal, Difgen Pharms, Ibsa, Nal Pharm, Noven Pharms Inc, Pharmobedient, Fougera Pharms, Hikma, Padagis Us, Pai Holdings Pharm, Zydus Lifesciences, Abbott, Abraxis Pharm, Afaxys, Am Regent, Aspiro, B Braun Medical, Bel Mar, Dell Labs, Elkins Sinn, Epic Pharma Llc, Eugia Pharma, Fresenius Kabi Usa, Gd Searle Llc, Hospira, Huons, Intl Medication, Luitpold, Lyphomed, Mankind Pharma, Miles, Neocubes Pharma, Spectra Mdcl Devices, Watson Labs, West-ward Pharms Int, Wyeth Ayerst, Yiling, Sentiss, Watson Labs Inc, Rubicon Research, Lannett Co Inc, Novitium Pharma, Paco, The J Molner, Baxter Hlthcare, B Braun, Eastman Kodak, Empi, Extrovis, Actavis Mid Atlantic, and Chartwell Molecular. and is included in one hundred and forty-seven NDAs.
The generic ingredient in LIDOCAINE is epinephrine; lidocaine hydrochloride. There are twenty-one drug master file entries for this compound. Fifteen suppliers are listed for this compound. Additional details are available on the epinephrine; lidocaine hydrochloride profile page.
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for LIDOCAINE?
- What are the global sales for LIDOCAINE?
- What is Average Wholesale Price for LIDOCAINE?
Summary for LIDOCAINE
| US Patents: | 0 |
| Applicants: | 71 |
| NDAs: | 147 |
| Finished Product Suppliers / Packagers: | 36 |
| Raw Ingredient (Bulk) Api Vendors: | 104 |
| Clinical Trials: | 2,102 |
| Patent Applications: | 4,213 |
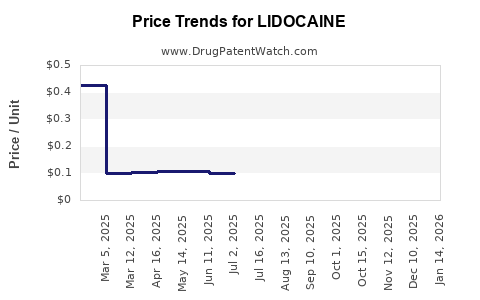
| Drug Prices: | Drug price information for LIDOCAINE |
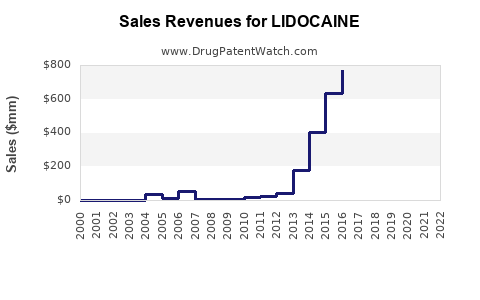
| Drug Sales Revenues: | Drug sales revenues for LIDOCAINE |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for LIDOCAINE |
| What excipients (inactive ingredients) are in LIDOCAINE? | LIDOCAINE excipients list |
| DailyMed Link: | LIDOCAINE at DailyMed |
Recent Clinical Trials for LIDOCAINE
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Vanderbilt University Medical Center | PHASE4 |
| Assiut University | NA |
| Universidad Industrial de Santander | PHASE1 |
Pharmacology for LIDOCAINE
| Drug Class | Amide Local Anesthetic Antiarrhythmic |
| Physiological Effect | Local Anesthesia |
Medical Subject Heading (MeSH) Categories for LIDOCAINE
Anatomical Therapeutic Chemical (ATC) Classes for LIDOCAINE
US Patents and Regulatory Information for LIDOCAINE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hospira | LIDOCAINE HYDROCHLORIDE | lidocaine hydrochloride | INJECTABLE;INJECTION | 083158-001 | Approved Prior to Jan 1, 1982 | AP | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Rising | LIDOCAINE HYDROCHLORIDE | lidocaine hydrochloride | INJECTABLE;INJECTION | 091056-002 | Dec 8, 2010 | DISCN | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Abraxis Pharm | LIDOCAINE HYDROCHLORIDE | lidocaine hydrochloride | INJECTABLE;INJECTION | 080420-002 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Hikma | LIDOCAINE AND PRILOCAINE | lidocaine; prilocaine | CREAM;TOPICAL | 076290-001 | Sep 25, 2003 | DISCN | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Hospira | LIDOCAINE HYDROCHLORIDE IN PLASTIC CONTAINER | lidocaine hydrochloride | INJECTABLE;INJECTION | 088325-001 | Jul 31, 1984 | AP | RX | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Hospira | LIDOCAINE HYDROCHLORIDE AND EPINEPHRINE | epinephrine; lidocaine hydrochloride | INJECTABLE;INJECTION | 089650-001 | Jun 21, 1988 | DISCN | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Market Dynamics and Financial Trajectory for Lidocaine: An In-Depth Analysis
More… ↓
