Loratadine - Generic Drug Details
✉ Email this page to a colleague
What are the generic drug sources for loratadine and what is the scope of patent protection?
Loratadine
is the generic ingredient in eleven branded drugs marketed by Bayer Healthcare Llc, Aurobindo Pharma, Bionpharma, Marksans Pharma, Strides Pharma, Taro, Aurobindo Pharma Ltd, Hetero Labs Ltd Iii, Lannett Co Inc, Perrigo, Pharm Assoc, Ranbaxy Labs Ltd, Teva, Wockhardt Bio Ag, Perrigo Pharma Intl, Sun Pharm, Fdn Consumer, Actavis Labs Fl Inc, Glaxosmithkline, Rubicon, Tenshi, Sun Pharm Inds Ltd, Apotex Inc, Granules, Guardian Drug, Hetero Labs Ltd V, Mylan, Pld Acquisitions Llc, Unique Pharm, Heritage Pharma, and P And L, and is included in fifty-two NDAs. Additional information is available in the individual branded drug profile pages.There are thirty-nine drug master file entries for loratadine. One hundred and fifty suppliers are listed for this compound.
Summary for loratadine
| US Patents: | 0 |
| Tradenames: | 11 |
| Applicants: | 31 |
| NDAs: | 52 |
| Drug Master File Entries: | 39 |
| Finished Product Suppliers / Packagers: | 150 |
| Raw Ingredient (Bulk) Api Vendors: | 148 |
| Clinical Trials: | 62 |
| Patent Applications: | 6,317 |
| Formulation / Manufacturing: | see details |
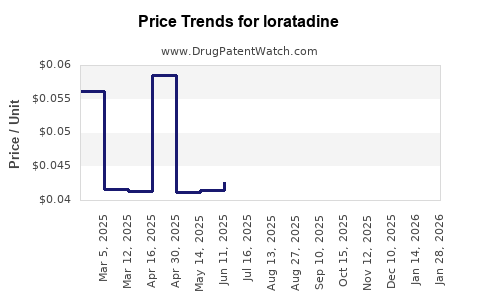
| Drug Prices: | Drug price trends for loratadine |
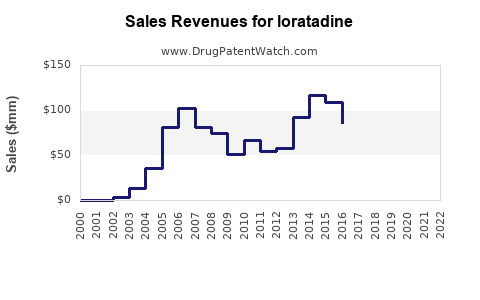
| Drug Sales Revenues: | Drug sales revenues for loratadine |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for loratadine |
| What excipients (inactive ingredients) are in loratadine? | loratadine excipients list |
| DailyMed Link: | loratadine at DailyMed |
Recent Clinical Trials for loratadine
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| AHS Cancer Control Alberta | Phase 2 |
| King Edward Medical University | Phase 4 |
| Ain Shams University | Phase 3 |
Medical Subject Heading (MeSH) Categories for loratadine
Anatomical Therapeutic Chemical (ATC) Classes for loratadine
US Patents and Regulatory Information for loratadine
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Perrigo | LORATADINE | loratadine | TABLET;ORAL | 076301-001 | Jun 25, 2004 | OTC | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Sun Pharm | LORATADINE | loratadine | TABLET, CHEWABLE;ORAL | 210088-001 | Apr 16, 2018 | OTC | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Wockhardt Bio Ag | LORATADINE | loratadine | SYRUP;ORAL | 075815-001 | Aug 20, 2004 | OTC | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Pharm Assoc | LORATADINE | loratadine | SYRUP;ORAL | 075565-001 | Oct 5, 2004 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Perrigo Pharma Intl | LORATADINE AND PSEUDOEPHEDRINE SULFATE | loratadine; pseudoephedrine sulfate | TABLET, EXTENDED RELEASE;ORAL | 075989-001 | Mar 4, 2004 | OTC | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Aurobindo Pharma Ltd | LORATADINE | loratadine | TABLET;ORAL | 208314-001 | Apr 16, 2018 | OTC | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for loratadine
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Bayer Healthcare Llc | CLARITIN | loratadine | TABLET;ORAL | 019658-002 | Nov 27, 2002 | ⤷ Try a Trial | ⤷ Try a Trial |
| Bayer Healthcare Llc | CLARITIN | loratadine | SYRUP;ORAL | 020641-002 | Nov 27, 2002 | ⤷ Try a Trial | ⤷ Try a Trial |
| Bayer Healthcare Llc | CLARITIN REDITABS | loratadine | TABLET, ORALLY DISINTEGRATING;ORAL | 020704-002 | Nov 27, 2002 | ⤷ Try a Trial | ⤷ Try a Trial |
| Bayer Healthcare Llc | CLARITIN | loratadine | SYRUP;ORAL | 020641-002 | Nov 27, 2002 | ⤷ Try a Trial | ⤷ Try a Trial |
| Bayer Healthcare Llc | CLARITIN | loratadine | TABLET;ORAL | 019658-002 | Nov 27, 2002 | ⤷ Try a Trial | ⤷ Try a Trial |
| Bayer Healthcare Llc | CLARITIN REDITABS | loratadine | TABLET, ORALLY DISINTEGRATING;ORAL | 020704-002 | Nov 27, 2002 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |