TARO Company Profile
✉ Email this page to a colleague
What is the competitive landscape for TARO, and when can generic versions of TARO drugs launch?
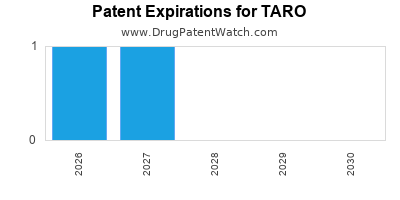
TARO has two hundred and fifty-five approved drugs.
There are eight US patents protecting TARO drugs. There are four tentative approvals on TARO drugs.
There are thirty-one patent family members on TARO drugs in twelve countries and one hundred and eighty-seven supplementary protection certificates in fourteen countries.
Summary for TARO
| International Patents: | 31 |
| US Patents: | 8 |
| Tradenames: | 134 |
| Ingredients: | 117 |
| NDAs: | 255 |
| Patent Litigation for TARO: | See patent lawsuits for TARO |
| PTAB Cases with TARO as petitioner: | See PTAB cases with TARO as petitioner |
Drugs and US Patents for TARO
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Taro | DICLOFENAC SODIUM | diclofenac sodium | SOLUTION;TOPICAL | 208098-001 | Sep 29, 2022 | AB | RX | No | No | ⤷ Sign Up | ⤷ Sign Up | ||||
| Taro | HYDROCORTISONE | hydrocortisone | CREAM;TOPICAL | 088799-001 | Nov 9, 1984 | AT | RX | No | No | ⤷ Sign Up | ⤷ Sign Up | ||||
| Taro | LAMOTRIGINE | lamotrigine | TABLET, FOR SUSPENSION;ORAL | 079204-002 | Feb 4, 2009 | AB | RX | No | No | ⤷ Sign Up | ⤷ Sign Up | ||||
| Taro | FLUOCINOLONE ACETONIDE | fluocinolone acetonide | OIL;TOPICAL | 209336-001 | May 19, 2016 | AT | RX | No | No | ⤷ Sign Up | ⤷ Sign Up | ||||
| Taro | IBUPROFEN | ibuprofen | SUSPENSION;ORAL | 209204-001 | Jun 23, 2017 | AB | RX | No | No | ⤷ Sign Up | ⤷ Sign Up | ||||
| Taro | MEPROBAMATE | meprobamate | TABLET;ORAL | 200998-001 | May 23, 2011 | DISCN | No | No | ⤷ Sign Up | ⤷ Sign Up | |||||
| Taro | CIPROFLOXACIN HYDROCHLORIDE | ciprofloxacin hydrochloride | TABLET;ORAL | 076912-002 | Oct 6, 2004 | DISCN | No | No | ⤷ Sign Up | ⤷ Sign Up | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for TARO
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Taro | FLO-PRED | prednisolone acetate | SUSPENSION;ORAL | 022067-001 | Jan 17, 2008 | 6,656,482 | ⤷ Sign Up |
| Taro | FLO-PRED | prednisolone acetate | SUSPENSION;ORAL | 022067-002 | Jan 17, 2008 | 6,102,254 | ⤷ Sign Up |
| Taro | FLO-PRED | prednisolone acetate | SUSPENSION;ORAL | 022067-002 | Jan 17, 2008 | 6,399,079 | ⤷ Sign Up |
| Taro | FLO-PRED | prednisolone acetate | SUSPENSION;ORAL | 022067-001 | Jan 17, 2008 | 6,102,254 | ⤷ Sign Up |
| Taro | FLO-PRED | prednisolone acetate | SUSPENSION;ORAL | 022067-002 | Jan 17, 2008 | 6,656,482 | ⤷ Sign Up |
| Taro | PLIAGLIS | lidocaine; tetracaine | CREAM;TOPICAL | 021717-001 | Jun 29, 2006 | 5,919,479 | ⤷ Sign Up |
| Taro | FLO-PRED | prednisolone acetate | SUSPENSION;ORAL | 022067-002 | Jan 17, 2008 | 5,881,926 | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for TARO drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Topical Lotion | 0.5% | ➤ Subscribe | 2011-03-16 |
| ➤ Subscribe | Topical Spray | 0.25% | ➤ Subscribe | 2013-12-18 |
International Patents for TARO Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Canada | 2616112 | ⤷ Sign Up |
| China | 102834096 | ⤷ Sign Up |
| Japan | 2013032405 | ⤷ Sign Up |
| European Patent Office | 2523660 | ⤷ Sign Up |
| Canada | 2822220 | ⤷ Sign Up |
| World Intellectual Property Organization (WIPO) | 2007005988 | ⤷ Sign Up |
| Australia | 2006278620 | ⤷ Sign Up |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for TARO Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0463756 | C990005 | Netherlands | ⤷ Sign Up | PRODUCT NAME: SILDENAFIL, DESGEWENST IN DE VORM VAN EEN FARMACEUTISCH AAN- VAARDBAAR ZOUT, IN HET BIJZONDER SILDENAFIL CITRAAT; NATL REGISTRATION NO/DATE: EU/1/98/077/001-012 19980914; FIRST REGISTRATION: CH 54642 19980622 |
| 2822954 | 18C1035 | France | ⤷ Sign Up | PRODUCT NAME: BICTEGRAVIR OU UN SEL PHARMACEUTIQUEMENT ACCEPTABLE,EN PARTICULIER BICTEGRAVIR DE SODIUM; REGISTRATION NO/DATE: EU/1/18/1289 20180625 |
| 3043773 | 2022C/520 | Belgium | ⤷ Sign Up | PRODUCT NAME: MOMETASONE OF EEN ZOUT HIERVAN EN OLOPATADINE OF EEN ZOUT HIERVAN; AUTHORISATION NUMBER AND DATE: BE595626 20220203 |
| 1948158 | 16C0018 | France | ⤷ Sign Up | PRODUCT NAME: SACUBITRIL/VALSARTAN,SOUS FORME DE COMPLEXE SODIQUE SACUBITRIL VALSARTAN,C'EST-A-DIRE DE (3-((1S,3R)-1-BIPHENYL-4-YLMETHYL-3-ETHOXYCARBONYL-1-BUTYLCARBAMOYL) PROPIONATE-(S)-3'-METHYL-2'-(PENTANOY(2''-(TETRAZOL-5-YLATE)BIPHENYL-4'-YLMETHYL)AMINO)BUTYRATE)DE TRISODIUM HEMIPENTAHYDRATE; REGISTRATION NO/DATE: EU/1/15/1058 20151123 |
| 2380576 | SPC/GB20/050 | United Kingdom | ⤷ Sign Up | PRODUCT NAME: DEOXYCHOLIC ACID SODIUM SALT; REGISTERED: UK PL 45496/0009 20170526 |
| 0124495 | SPC/GB01/006 | United Kingdom | ⤷ Sign Up | PRODUCT NAME: ESOMEPRAZOLE AS MAGNESIUM TRIHYDRATE; REGISTERED: SE 15945 20000310; SE 15946 20000310; UK PL 17901/0068-0069 20000727 |
| 0480717 | 98C0022 | France | ⤷ Sign Up | PRODUCT NAME: MONTELUKAST SODIUM; REGISTRATION NO/DATE IN FRANCE: NL 23 133 DU 19980320; REGISTRATION NO/DATE AT EEC: 13 651 DU 19970825 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.