GLAXOSMITHKLINE Company Profile
✉ Email this page to a colleague
What is the competitive landscape for GLAXOSMITHKLINE, and when can generic versions of GLAXOSMITHKLINE drugs launch?
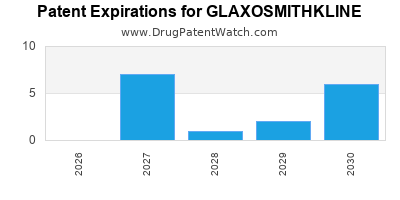
GLAXOSMITHKLINE has one hundred and forty-nine approved drugs.
There are thirty-one US patents protecting GLAXOSMITHKLINE drugs.
There are seven hundred and two patent family members on GLAXOSMITHKLINE drugs in fifty-nine countries and one hundred and eighty-five supplementary protection certificates in twenty countries.
Summary for GLAXOSMITHKLINE
| International Patents: | 702 |
| US Patents: | 31 |
| Tradenames: | 104 |
| Ingredients: | 90 |
| NDAs: | 149 |
| Drug Master File Entries: | 8 |
| Patent Litigation for GLAXOSMITHKLINE: | See patent lawsuits for GLAXOSMITHKLINE |
| PTAB Cases with GLAXOSMITHKLINE as petitioner: | See PTAB cases with GLAXOSMITHKLINE as petitioner |
Drugs and US Patents for GLAXOSMITHKLINE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glaxosmithkline | RELENZA | zanamivir | POWDER;INHALATION | 021036-001 | Jul 26, 1999 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | |||||
| Glaxosmithkline | ZEJULA | niraparib tosylate | CAPSULE;ORAL | 208447-001 | Mar 27, 2017 | DISCN | Yes | No | ⤷ Sign Up | ⤷ Sign Up | |||||
| Glaxosmithkline | WELLBUTRIN SR | bupropion hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 020358-001 | Oct 4, 1996 | DISCN | No | No | ⤷ Sign Up | ⤷ Sign Up | |||||
| Glaxosmithkline | TICAR | ticarcillin disodium | INJECTABLE;INJECTION | 050497-002 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Sign Up | ⤷ Sign Up | |||||
| Glaxosmithkline | AGENERASE | amprenavir | SOLUTION;ORAL | 021039-001 | Apr 15, 1999 | DISCN | Yes | No | ⤷ Sign Up | ⤷ Sign Up | |||||
| Glaxosmithkline | OJJAARA | momelotinib dihydrochloride | TABLET;ORAL | 216873-001 | Sep 15, 2023 | RX | Yes | No | 8,486,941 | ⤷ Sign Up | Y | Y | ⤷ Sign Up | ||
| Glaxosmithkline | EMPRACET W/ CODEINE PHOSPHATE #4 | acetaminophen; codeine phosphate | TABLET;ORAL | 083951-002 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Sign Up | ⤷ Sign Up | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for GLAXOSMITHKLINE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Glaxosmithkline | IMITREX | sumatriptan | SPRAY;NASAL | 020626-002 | Aug 26, 1997 | 5,307,953*PED | ⤷ Sign Up |
| Glaxosmithkline | VALTREX | valacyclovir hydrochloride | TABLET;ORAL | 020487-001 | Jun 23, 1995 | 5,879,706*PED | ⤷ Sign Up |
| Glaxosmithkline | VENTOLIN HFA | albuterol sulfate | AEROSOL, METERED;INHALATION | 020983-001 | Apr 19, 2001 | 7,107,986*PED | ⤷ Sign Up |
| Glaxosmithkline | ZANTAC 300 | ranitidine hydrochloride | CAPSULE;ORAL | 020095-002 | Mar 8, 1994 | 4,521,431*PED | ⤷ Sign Up |
| Glaxosmithkline | ZYBAN | bupropion hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 020711-002 | May 14, 1997 | 5,427,798 | ⤷ Sign Up |
| Glaxosmithkline | MALARONE | atovaquone; proguanil hydrochloride | TABLET;ORAL | 021078-001 | Jul 14, 2000 | 6,166,046*PED | ⤷ Sign Up |
| Glaxosmithkline | IMITREX STATDOSE | sumatriptan succinate | INJECTABLE;SUBCUTANEOUS | 020080-003 | Dec 23, 1996 | 4,816,470*PED | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for GLAXOSMITHKLINE drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Extended-release Tablets | 8 mg | ➤ Subscribe | 2008-11-03 |
| ➤ Subscribe | Extended-release Tablets | 12 mg | ➤ Subscribe | 2009-02-05 |
| ➤ Subscribe | Extended-release Tablets | 6 mg | ➤ Subscribe | 2009-07-22 |
| ➤ Subscribe | Tablets | 150 mg | ➤ Subscribe | 2007-10-30 |
| ➤ Subscribe | Orally Disintegrating Tablets | 25 mg, 50 mg, 100 mg, and 200 mg | ➤ Subscribe | 2009-12-21 |
| ➤ Subscribe | Injection | 6 mg/0.5 mL, 0.5 mL (prefilled syringes) | ➤ Subscribe | 2006-05-09 |
| ➤ Subscribe | Tablets | 3 mg, 4 mg and 5 mg | ➤ Subscribe | 2005-02-04 |
| ➤ Subscribe | Tablets | 250 mg/100 mg | ➤ Subscribe | 2009-04-03 |
| ➤ Subscribe | Extended-release Capsules | 325 mg | ➤ Subscribe | 2006-11-07 |
| ➤ Subscribe | Oral Suspension | 750 mg/5 mL | ➤ Subscribe | 2009-10-20 |
| ➤ Subscribe | Extended-release Tablets | 4 mg | ➤ Subscribe | 2008-10-31 |
| ➤ Subscribe | Extended-release Tablets | 3 mg | ➤ Subscribe | 2009-01-08 |
| ➤ Subscribe | Extended-release Tablets | 2 mg | ➤ Subscribe | 2008-10-14 |
| ➤ Subscribe | Extended-release Tablets | 3 mg | ➤ Subscribe | 2009-01-08 |
| ➤ Subscribe | Injection | 6 mg/0.5 mL, 0.5 mL vials | ➤ Subscribe | 2004-10-25 |
| ➤ Subscribe | Tablets | 0.25 mg, 0.5 mg, 1 mg and 2 mg | ➤ Subscribe | 2004-12-22 |
| ➤ Subscribe | Tablets | 100 mg | ➤ Subscribe | 2007-10-31 |
| ➤ Subscribe | Tablets | 62.5 mg/25 mg | ➤ Subscribe | 2010-09-14 |
| ➤ Subscribe | Extended-release Capsules | 225 mg and 425 mg | ➤ Subscribe | 2006-10-11 |
| ➤ Subscribe | Extended-release Tablets | 25 mg, 50 mg, 100 mg, 200 mg, 250 mg, and 300 mg | ➤ Subscribe | 2014-02-12 |
International Patents for GLAXOSMITHKLINE Drugs
Supplementary Protection Certificates for GLAXOSMITHKLINE Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1425001 | PA2014019 | Lithuania | ⤷ Sign Up | PRODUCT NAME: VILANTEROLUM; REGISTRATION NO/DATE: EU/1/13/886/001-006 20131113 |
| 1425001 | C 2014 016 | Romania | ⤷ Sign Up | PRODUCT NAME: VILANTEROL SAU O SARE SAU SOLVAT AL ACESTUIA; NATIONAL AUTHORISATION NUMBER: EU/1/13/886/001, EU/1/13/886/002, EU/1/13/886/003, EU/1/13/886/004, EU/1/13/886/005, EU/1/13/886/006; DATE OF NATIONAL AUTHORISATION: 20131113; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/13/886/001, EU/1/13/886/002, EU/1/13/886/003, EU/1/13/886/004, EU/1/13/886/005, EU/1/13/886/006; DATE OF FIRST AUTHORISATION IN EEA: 20131113 |
| 1480644 | SPC/GB17/004 | United Kingdom | ⤷ Sign Up | PRODUCT NAME: COMBINATION OF CEFTAZIDIME, OR A SALT THEREOF, AND AVIBACTAM, OR A SALT THEREOF; REGISTERED: UK EU/1/16/1109/001 20160628 |
| 2316456 | 122017000109 | Germany | ⤷ Sign Up | PRODUCT NAME: NALTREXON ODER EIN PHARMAZEUTISCH AKZEPTABLES SALZ DAVON, INSBESONDERE NALTREXONHYDROCHLORID, UND BUPROPION ODER EIN PHARMAZEUTISCH AKZEPTABLES SALZ DAVON, INSBESONDERE BUPROPIONHYDROCHLORID; REGISTRATION NO/DATE: EU/1/14/988 20150326 |
| 2316456 | 349 22-2017 | Slovakia | ⤷ Sign Up | PRODUCT NAME: KOMBINACIA NALTREXONU VO VSETKYCH FORMACH CHRANENYCH ZAKLADNYM PATENTOM A BUPROPIONU VO VSETKYCH FORMACH CHRANENYCH ZAKLADNYM PATENTOM; REGISTRATION NO/DATE: EU/1/14/988 20150330 |
| 0299602 | SPC/GB96/040 | United Kingdom | ⤷ Sign Up | PRODUCT NAME: ROPINIROLE, OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF, IN PARTICULAR THE HYDROCHLORIDE SALT; REGISTERED: UK 10592/0085 19960702 |
| 0984957 | C300517 | Netherlands | ⤷ Sign Up | PRODUCT NAME: ACETYLSALICYLZUUR EN ESOMEPRAZOLMAGNESIUMTRIHYDRAAT; NAT. REGISTRATION NO/DATE: RVG 107516 20110912; FIRST REGISTRATION: 540235954023675402375 2011120812 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.