GILEAD SCIENCES INC Company Profile
✉ Email this page to a colleague
What is the competitive landscape for GILEAD SCIENCES INC, and what generic alternatives to GILEAD SCIENCES INC drugs are available?
GILEAD SCIENCES INC has twenty-three approved drugs.
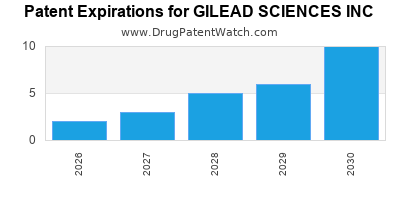
There are eighty US patents protecting GILEAD SCIENCES INC drugs.
There are two thousand two hundred and forty-four patent family members on GILEAD SCIENCES INC drugs in sixty-six countries and three hundred and seventy supplementary protection certificates in nineteen countries.
Summary for GILEAD SCIENCES INC
| International Patents: | 2244 |
| US Patents: | 80 |
| Tradenames: | 18 |
| Ingredients: | 18 |
| NDAs: | 23 |
| Drug Master File Entries: | 7 |
| Patent Litigation for GILEAD SCIENCES INC: | See patent lawsuits for GILEAD SCIENCES INC |
Drugs and US Patents for GILEAD SCIENCES INC
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gilead Sciences Inc | EPCLUSA | sofosbuvir; velpatasvir | TABLET;ORAL | 208341-002 | Mar 19, 2020 | RX | Yes | No | 11,116,783*PED | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Gilead Sciences Inc | HARVONI | ledipasvir; sofosbuvir | PELLETS;ORAL | 212477-002 | Aug 28, 2019 | RX | Yes | Yes | 8,334,270*PED | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Gilead Sciences Inc | HARVONI | ledipasvir; sofosbuvir | TABLET;ORAL | 205834-002 | Aug 28, 2019 | RX | Yes | No | 8,735,372*PED | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Gilead Sciences Inc | SUNLENCA | lenacapavir sodium | SOLUTION;SUBCUTANEOUS | 215973-001 | Dec 22, 2022 | RX | Yes | Yes | 10,654,827 | ⤷ Sign Up | ⤷ Sign Up | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for GILEAD SCIENCES INC
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Gilead Sciences Inc | GENVOYA | cobicistat; elvitegravir; emtricitabine; tenofovir alafenamide fumarate | TABLET;ORAL | 207561-001 | Nov 5, 2015 | 6,642,245*PED | ⤷ Sign Up |
| Gilead Sciences Inc | VIREAD | tenofovir disoproxil fumarate | TABLET;ORAL | 021356-001 | Oct 26, 2001 | 6,043,230*PED | ⤷ Sign Up |
| Gilead Sciences Inc | COMPLERA | emtricitabine; rilpivirine hydrochloride; tenofovir disoproxil fumarate | TABLET;ORAL | 202123-001 | Aug 10, 2011 | 7,067,522 | ⤷ Sign Up |
| Gilead Sciences Inc | VIREAD | tenofovir disoproxil fumarate | TABLET;ORAL | 021356-004 | Jan 18, 2012 | 5,977,089*PED | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for GILEAD SCIENCES INC drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Tablets | 150 mg, 200 mg, and 250 mg | ➤ Subscribe | 2012-05-17 |
| ➤ Subscribe | Tablets | 150 mg, 150 mg, 200 mg, 300 mg | ➤ Subscribe | 2018-10-04 |
| ➤ Subscribe | Tablets | 300 mg | ➤ Subscribe | 2010-01-26 |
| ➤ Subscribe | Tablets | 200 mg/25 mg/300 mg | ➤ Subscribe | 2015-05-20 |
| ➤ Subscribe | Tablets | 150 mg | ➤ Subscribe | 2015-12-09 |
International Patents for GILEAD SCIENCES INC Drugs
Supplementary Protection Certificates for GILEAD SCIENCES INC Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2487163 | 93353 | Luxembourg | ⤷ Sign Up | PRODUCT NAME: COBICISTAT OU UN SEL PHARMACEUTIQUEMENT ACCEPTABLE DE CELUI-CI ET ATAZANAVIR OU UN SEL PHARMACEUTIQUEMENT ACCEPTABLE DE CELUI-CI, EN PARTICULIER LE SULFATE D'ATAZANAVIR; AUTHORISATION NUMBER AND DATE: EU/1/15/1025 |
| 1663240 | 92855 | Luxembourg | ⤷ Sign Up | PRODUCT NAME: COMBINAISON DE RILPIVIRINE OU UNE FORME THERAPEUTIQUE EQUIVALENTE QUI EN DERIVE TELLE QUE PROTEGEE PAR LE BREVET DE BASE, TEL QU'UN SEL PHARMACEUTIQUEMENT ACCEPTABLE DE RILPIVIRINE, INCLUANT LE SEL CHLORHYDRATE DE RILPIVIRINE , TENOFOVIR, EN PARTICULIER LE FUMARATE DE TENOFOVIR DISOPROXIL, ET L'EMTRICITABINE. |
| 1564210 | 132013902209844 | Italy | ⤷ Sign Up | PRODUCT NAME: COMPRENDENTE IL PRODOTTO ELVITEGRAVIR COME UNO DEI PRINCIPI ATTIV(STRIBILD); AUTHORISATION NUMBER(S) AND DATE(S): EU/1/13/830/001-002, 20130524 |
| 1564210 | 489 | Finland | ⤷ Sign Up | |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.