ASTRAZENECA Company Profile
✉ Email this page to a colleague
What is the competitive landscape for ASTRAZENECA, and what generic alternatives to ASTRAZENECA drugs are available?
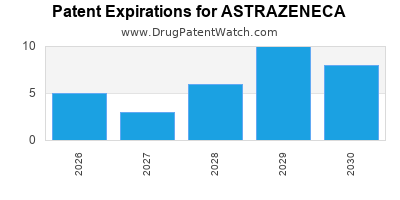
ASTRAZENECA has ninety-three approved drugs.
There are one hundred and twenty-five US patents protecting ASTRAZENECA drugs. There is one tentative approval on ASTRAZENECA drugs.
There are two thousand two hundred and three patent family members on ASTRAZENECA drugs in seventy countries and three hundred and forty-six supplementary protection certificates in twenty countries.
Summary for ASTRAZENECA
| International Patents: | 2203 |
| US Patents: | 125 |
| Tradenames: | 66 |
| Ingredients: | 60 |
| NDAs: | 93 |
| Patent Litigation for ASTRAZENECA: | See patent lawsuits for ASTRAZENECA |
| PTAB Cases with ASTRAZENECA as patent owner: | See PTAB cases with ASTRAZENECA as patent owner |
Drugs and US Patents for ASTRAZENECA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Astrazeneca Ab | BYDUREON BCISE | exenatide synthetic | SUSPENSION, EXTENDED RELEASE;SUBCUTANEOUS | 209210-001 | Oct 20, 2017 | RX | Yes | Yes | 8,906,851*PED | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Astrazeneca | BRILINTA | ticagrelor | TABLET;ORAL | 022433-002 | Sep 3, 2015 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | |||||
| Astrazeneca | EPANOVA | omega-3-carboxylic acids | CAPSULE;ORAL | 205060-001 | May 5, 2014 | DISCN | Yes | No | 8,383,678 | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Astrazeneca | TRUQAP | capivasertib | TABLET;ORAL | 218197-002 | Nov 16, 2023 | RX | Yes | Yes | 10,059,714 | ⤷ Sign Up | Y | Y | ⤷ Sign Up | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for ASTRAZENECA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Astrazeneca | PULMICORT RESPULES | budesonide | SUSPENSION;INHALATION | 020929-003 | Aug 8, 2000 | 4,787,536*PED | ⤷ Sign Up |
| Astrazeneca | DALIRESP | roflumilast | TABLET;ORAL | 022522-002 | Jan 23, 2018 | 8,431,154 | ⤷ Sign Up |
| Astrazeneca Ab | XIGDUO XR | dapagliflozin; metformin hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 205649-001 | Oct 29, 2014 | 6,936,590 | ⤷ Sign Up |
| Astrazeneca | PULMICORT | budesonide | POWDER, METERED;INHALATION | 020441-002 | Jun 24, 1997 | 4,668,218*PED | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for ASTRAZENECA drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Delayed-release | 20 mg and 40 mg | ➤ Subscribe | 2005-08-05 |
| ➤ Subscribe | Tablets | 100 mg, 200 mg and 300 mg | ➤ Subscribe | 2006-02-21 |
| ➤ Subscribe | For Injection | 20 mg/vial and 40 mg/vial | ➤ Subscribe | 2009-11-23 |
| ➤ Subscribe | Extended-release Tablets | 5 mg/500 mg, 2.5 mg/1000 mg, and 5 mg/1000 mg | ➤ Subscribe | 2013-07-31 |
| ➤ Subscribe | Inhalation Suspension | 0.25 mg/2 mL and 0.5 mg/2 mL | ➤ Subscribe | 2005-09-15 |
| ➤ Subscribe | Tablets | 2.5 mg and 5 mg | ➤ Subscribe | 2013-07-31 |
| ➤ Subscribe | Delayed-release Capsules | 20 mg | ➤ Subscribe | 2007-03-19 |
| ➤ Subscribe | Extended-release Tablets | 400 mg | ➤ Subscribe | 2008-06-18 |
| ➤ Subscribe | Tablets | 200 mg and 300 mg | ➤ Subscribe | 2008-06-12 |
| ➤ Subscribe | Tablets | 90 mg | ➤ Subscribe | 2015-07-20 |
| ➤ Subscribe | Tablets | 500 mcg | ➤ Subscribe | 2015-03-02 |
| ➤ Subscribe | Nasal Spray | 0.032 mg (32 mcg)/spray | ➤ Subscribe | 2007-05-14 |
| ➤ Subscribe | Tablets | 50 mg, 150 mg and 400 mg | ➤ Subscribe | 2007-02-12 |
| ➤ Subscribe | Delayed-release for Oral Suspension | 2.5 mg and 5 mg | ➤ Subscribe | 2018-09-24 |
| ➤ Subscribe | Injection | 250 mg/mL, 1.2 mL and 2.4 mL prefilled syringe | ➤ Subscribe | 2014-06-11 |
| ➤ Subscribe | Injection | 50 mg/mL, 2.5 mL and 5 mL syringe | ➤ Subscribe | 2009-10-01 |
| ➤ Subscribe | HydrochlorideExtended-release Tablets | 2.5 mg/1000 mg | ➤ Subscribe | 2018-10-29 |
| ➤ Subscribe | Inhalation Suspension | 1 mg/2 mL | ➤ Subscribe | 2010-05-28 |
| ➤ Subscribe | Delayed-release Tablets | 20 mg | ➤ Subscribe | 2012-03-30 |
| ➤ Subscribe | Extended-release Tablets | 150 mg | ➤ Subscribe | 2008-11-17 |
| ➤ Subscribe | Extended-release Tablets | 50 mg | ➤ Subscribe | 2008-10-17 |
| ➤ Subscribe | Tablets | 60 mg | ➤ Subscribe | 2015-09-30 |
| ➤ Subscribe | Tablets | 25 mg | ➤ Subscribe | 2005-08-12 |
International Patents for ASTRAZENECA Drugs
Supplementary Protection Certificates for ASTRAZENECA Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2673237 | CA 2019 00014 | Denmark | ⤷ Sign Up | PRODUCT NAME: NATRIUMZIRCONIUMCYCLOSILICAT; REG. NO/DATE: EU/1/17/1173 20180326 |
| 1685839 | 92292 | Luxembourg | ⤷ Sign Up | PRODUCT NAME: COMBINAISON D OXYCODONE EN TANT QUE COMPOSANT A ET DE NALOXONE EN TANT QUE COMPOSANT B SOUS TOUTES LES FORMES PROTEGES PAR LE BREVET DE BASE |
| 1856135 | 2090014-8 | Sweden | ⤷ Sign Up | PRODUCT NAME: FOSTAMATINIB OR A PHARMACEUTICALLY ACCEPTABLE SALT OF FOSTAMATINIB, OR A HYDRATE OR SOLVATE OF FOSTAMATINIB OR THE PH ARMACEUTICALLY ACCEPTABLE SALT OF FOSTAMATINIB, ESPECIALLY FOSTAMATINIB DISODIUM, OPTIONALLY IN FORM OF A HYDRATE; REG. NO/DATE: EU/1/19/1405 20200113 |
| 2203431 | CR 2015 00014 | Denmark | ⤷ Sign Up | PRODUCT NAME: DASABUVIR OR A SALT THEREOF, INCLUDING DASABUVIR SODIUM MONOHYDRATE; REG. NO/DATE: EU/1/14/983 20150119 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.