BYDUREON BCISE Drug Patent Profile
✉ Email this page to a colleague
When do Bydureon Bcise patents expire, and what generic alternatives are available?
Bydureon Bcise is a drug marketed by Astrazeneca Ab and is included in one NDA. There are eleven patents protecting this drug.
This drug has three hundred and seventy-eight patent family members in forty-nine countries.
The generic ingredient in BYDUREON BCISE is exenatide synthetic. There are seven drug master file entries for this compound. Two suppliers are listed for this compound. Additional details are available on the exenatide synthetic profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Bydureon Bcise
A generic version of BYDUREON BCISE was approved as exenatide synthetic by AMNEAL on November 19th, 2024.
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for BYDUREON BCISE?
- What are the global sales for BYDUREON BCISE?
- What is Average Wholesale Price for BYDUREON BCISE?
Summary for BYDUREON BCISE
| International Patents: | 378 |
| US Patents: | 11 |
| Applicants: | 1 |
| NDAs: | 1 |
| Clinical Trials: | 40 |
| Drug Prices: | Drug price information for BYDUREON BCISE |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for BYDUREON BCISE |
| What excipients (inactive ingredients) are in BYDUREON BCISE? | BYDUREON BCISE excipients list |
| DailyMed Link: | BYDUREON BCISE at DailyMed |
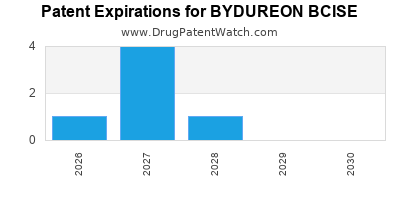
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for BYDUREON BCISE
Generic Entry Date for BYDUREON BCISE*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
SUSPENSION, EXTENDED RELEASE;SUBCUTANEOUS |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for BYDUREON BCISE
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Christopher D. Verrico | PHASE1 |
| The University of Texas Health Science Center, Houston | Phase 2 |
| University of Washington | Phase 3 |
US Patents and Regulatory Information for BYDUREON BCISE
BYDUREON BCISE is protected by eleven US patents and two FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of BYDUREON BCISE is ⤷ Get Started Free.
This potential generic entry date is based on patent 8,895,033.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Astrazeneca Ab | BYDUREON BCISE | exenatide synthetic | SUSPENSION, EXTENDED RELEASE;SUBCUTANEOUS | 209210-001 | Oct 20, 2017 | DISCN | Yes | No | 7,612,176*PED | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Astrazeneca Ab | BYDUREON BCISE | exenatide synthetic | SUSPENSION, EXTENDED RELEASE;SUBCUTANEOUS | 209210-001 | Oct 20, 2017 | DISCN | Yes | No | 8,906,851*PED | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Astrazeneca Ab | BYDUREON BCISE | exenatide synthetic | SUSPENSION, EXTENDED RELEASE;SUBCUTANEOUS | 209210-001 | Oct 20, 2017 | DISCN | Yes | No | 8,431,685*PED | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for BYDUREON BCISE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Astrazeneca Ab | BYDUREON BCISE | exenatide synthetic | SUSPENSION, EXTENDED RELEASE;SUBCUTANEOUS | 209210-001 | Oct 20, 2017 | 9,198,925 | ⤷ Get Started Free |
| Astrazeneca Ab | BYDUREON BCISE | exenatide synthetic | SUSPENSION, EXTENDED RELEASE;SUBCUTANEOUS | 209210-001 | Oct 20, 2017 | 6,414,126 | ⤷ Get Started Free |
| Astrazeneca Ab | BYDUREON BCISE | exenatide synthetic | SUSPENSION, EXTENDED RELEASE;SUBCUTANEOUS | 209210-001 | Oct 20, 2017 | 6,936,590 | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for BYDUREON BCISE
When does loss-of-exclusivity occur for BYDUREON BCISE?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 09289529
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 0918904
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 34525
Estimated Expiration: ⤷ Get Started Free
China
Patent: 2164597
Estimated Expiration: ⤷ Get Started Free
Patent: 4248623
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0201179
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 23410
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 41905
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 0299
Estimated Expiration: ⤷ Get Started Free
Patent: 1170413
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 41905
Estimated Expiration: ⤷ Get Started Free
Patent: 85837
Estimated Expiration: ⤷ Get Started Free
Finland
Patent: 41905
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 50125
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 1231
Patent: הרכבים בעלי שחרור מושהה עם נשאים שאינם מימיים (Sustained release formulations using non-aqueous carriers)
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 44735
Estimated Expiration: ⤷ Get Started Free
Patent: 51243
Estimated Expiration: ⤷ Get Started Free
Patent: 12502056
Estimated Expiration: ⤷ Get Started Free
Patent: 15110637
Patent: 非水性担体を用いた徐放性製剤 (SUSTAINED RELEASE FORMULATION USING NON-AQUEOUS CARRIER)
Estimated Expiration: ⤷ Get Started Free
Lithuania
Patent: 41905
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 2189
Patent: FORMULACIONES DE LIBERACIÓN SOSTENIDA USANDO PORTADORES NO ACUOSOS. (SUSTAINED RELEASE FORMULATIONS USING NON-AQUEOUS CARRIERS.)
Estimated Expiration: ⤷ Get Started Free
Patent: 11002398
Patent: FORMULACIONES DE LIBERACION SOSTENIDA USANDO PORTADORES NO ACUOSOS. (SUSTAINED RELEASE FORMULATIONS USING NON-AQUEOUS CARRIERS.)
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 4997
Patent: Sustained release formulations using non-aqueous carriers
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 41905
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 41905
Estimated Expiration: ⤷ Get Started Free
San Marino
Patent: 02000405
Estimated Expiration: ⤷ Get Started Free
Singapore
Patent: 201703039S
Patent: SUSTAINED RELEASE FORMULATIONS USING NON-AQUEOUS CARRIERS
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 41905
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1760953
Estimated Expiration: ⤷ Get Started Free
Patent: 110050540
Patent: SUSTAINED RELEASE FORMULATIONS USING NON-AQUEOUS CARRIERS
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 09178
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering BYDUREON BCISE around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| China | 104248623 | ⤷ Get Started Free | |
| New Zealand | 589202 | ⤷ Get Started Free | |
| Canada | 2560874 | MICROCAPSULES A LIBERATION PROLONGEE, A BASE DE POLY (LACTIDE-CO-GLYCOLIDE), CONTENANT UN POLYPEPTIDE ET UN SUCRE (POLY (LACTIDE-CO-GLYCOLIDE)-BASED SUSTAINED RELEASE MICROCAPSULES COMPRISING A POLYPEPTIDE AND A SUGAR) | ⤷ Get Started Free |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for BYDUREON BCISE
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1506211 | 179 5017-2014 | Slovakia | ⤷ Get Started Free | PRODUCT NAME: KOMBINACIA DAPAGLIFLOZINU ALEBO JEHO FARMACEUTICKY PRIJATELNYCH SOLI A METFORMINU ALEBO JEHO FARMACEUTICKY PRIJATELNYCH SOLI; REGISTRATION NO/DATE: EU/1/13/900/001 - EU/1/13/900/012 20140116 |
| 1506211 | 122014000071 | Germany | ⤷ Get Started Free | PRODUCT NAME: KOMBINATION VON DAPAGLIFLOZIN ODER EINEM PHARMAZEUTISCH VERTRAEGLICHEN SALZ DAVON, UND METFORMIN ODER EINEM PHARMAZEUTISCH VERTRAEGLICHEN SALZ DAVON, GESCHUETZT DURCH DAS GRUNDPATENT EP 1 506 211; REGISTRATION NO/DATE: EU/1/13/900 20140116 |
| 1506211 | C01506211/01 | Switzerland | ⤷ Get Started Free | PRODUCT NAME: DAPAGLIFLOZIN; REGISTRATION NO/DATE: SWISSMEDIC 65176 19.08.2014 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for BYDUREON BCISE
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
