ASTRAZENECA AB Company Profile
✉ Email this page to a colleague
What is the competitive landscape for ASTRAZENECA AB, and what generic alternatives to ASTRAZENECA AB drugs are available?
ASTRAZENECA AB has twelve approved drugs.
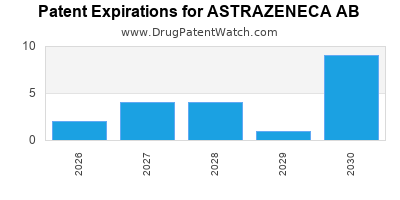
There are fifty-five US patents protecting ASTRAZENECA AB drugs. There is one tentative approval on ASTRAZENECA AB drugs.
There are eight hundred and ninety-six patent family members on ASTRAZENECA AB drugs in fifty-four countries and one hundred and forty-two supplementary protection certificates in nineteen countries.
Summary for ASTRAZENECA AB
| International Patents: | 896 |
| US Patents: | 55 |
| Tradenames: | 13 |
| Ingredients: | 10 |
| NDAs: | 12 |
| Drug Master File Entries: | 2 |
| Patent Litigation for ASTRAZENECA AB: | See patent lawsuits for ASTRAZENECA AB |
| PTAB Cases with ASTRAZENECA AB as patent owner: | See PTAB cases with ASTRAZENECA AB as patent owner |
Drugs and US Patents for ASTRAZENECA AB
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Astrazeneca Ab | BYDUREON BCISE | exenatide synthetic | SUSPENSION, EXTENDED RELEASE;SUBCUTANEOUS | 209210-001 | Oct 20, 2017 | RX | Yes | Yes | 9,238,076*PED | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Astrazeneca Ab | SYMLIN | pramlintide acetate | INJECTABLE;SUBCUTANEOUS | 021332-003 | Sep 25, 2007 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Astrazeneca Ab | QTERN | dapagliflozin; saxagliptin hydrochloride | TABLET;ORAL | 209091-002 | May 2, 2019 | RX | Yes | No | 7,919,598 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Astrazeneca Ab | BYDUREON BCISE | exenatide synthetic | SUSPENSION, EXTENDED RELEASE;SUBCUTANEOUS | 209210-001 | Oct 20, 2017 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Astrazeneca Ab | BYDUREON PEN | exenatide synthetic | FOR SUSPENSION, EXTENDED RELEASE;SUBCUTANEOUS | 022200-002 | Feb 28, 2014 | DISCN | Yes | No | 8,690,837*PED | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for ASTRAZENECA AB
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Astrazeneca Ab | SYMLIN | pramlintide acetate | INJECTABLE;SUBCUTANEOUS | 021332-003 | Sep 25, 2007 | 6,610,824 | ⤷ Try a Trial |
| Astrazeneca Ab | QTERNMET XR | dapagliflozin; metformin hydrochloride; saxagliptin hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 210874-004 | May 2, 2019 | 6,414,126 | ⤷ Try a Trial |
| Astrazeneca Ab | BYETTA | exenatide synthetic | INJECTABLE;SUBCUTANEOUS | 021773-001 | Apr 28, 2005 | 6,872,700 | ⤷ Try a Trial |
| Astrazeneca Ab | QTERNMET XR | dapagliflozin; metformin hydrochloride; saxagliptin hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 210874-003 | May 2, 2019 | 6,414,126 | ⤷ Try a Trial |
| Astrazeneca Ab | FARXIGA | dapagliflozin | TABLET;ORAL | 202293-001 | Jan 8, 2014 | 7,563,871 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for ASTRAZENECA AB drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Injection | 250 mg/mL, 1.2 mL and 2.4 mL prefilled syringe | ➤ Subscribe | 2014-06-11 |
| ➤ Subscribe | HydrochlorideExtended-release Tablets | 2.5 mg/1000 mg | ➤ Subscribe | 2018-10-29 |
| ➤ Subscribe | Extended-release Tablets | 5 mg/500 mg, 2.5 mg/1000 mg, and 5 mg/1000 mg | ➤ Subscribe | 2013-07-31 |
| ➤ Subscribe | Tablets | 2.5 mg and 5 mg | ➤ Subscribe | 2013-07-31 |
International Patents for ASTRAZENECA AB Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Poland | 2139494 | ⤷ Try a Trial |
| Japan | 6876734 | ⤷ Try a Trial |
| European Patent Office | 2139494 | ⤷ Try a Trial |
| Chile | 2007001915 | ⤷ Try a Trial |
| Hong Kong | 1068214 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for ASTRAZENECA AB Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0996459 | SZ 31/2007 | Austria | ⤷ Try a Trial | PRODUCT NAME: EXENATIDE |
| 1734971 | 16/2012 | Austria | ⤷ Try a Trial | PRODUCT NAME: EXENATID; REGISTRATION NO/DATE: EU/1/11/696/001-002 (MITTEILUNG) 20110623 |
| 1506211 | C01506211/02 | Switzerland | ⤷ Try a Trial | PRODUCT NAME: DAPAGLIFLOZIN + METFORMIN; REGISTRATION NO/DATE: SWISSMEDIC 65377 16.07.2015 |
| 1506211 | PA2014026 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: DAPAGLIFOZINUM + METFORMINUM; REGISTRATION NO/DATE: EU/1/13/900 20140116 |
| 1412357 | 132008901682802 | Italy | ⤷ Try a Trial | PRODUCT NAME: SITAGLIPTIN/METFORMINA CLORIDRATO(JANUMET, VELMETIA, EFFICIB); AUTHORISATION NUMBER(S) AND DATE(S): JANUMET:EU/1/08/455/001... 014; VELMETIA:EU/1/08/456/001...014;EFFICIB: EU/1/08/457/001....014, 20080716;58450-01;58450-02; 58450-03, 20080408 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.