Last updated: July 27, 2025
Introduction
Metronidazole, a nitroimidazole antibiotic and antiprotozoal agent, continues to underpin treatment protocols for various infectious diseases globally. Its long history of clinical utility, coupled with evolving market forces, shapes its current and projected market trajectory. As antibiotic resistance, regulatory shifts, and emerging alternatives influence the landscape, understanding the intricate market dynamics and financial outlook of metronidazole remains vital for stakeholders—including pharmaceutical manufacturers, healthcare providers, and investors.
Overview of Metronidazole
Initially synthesized in the 1950s, metronidazole gained rapid acceptance for treating protozoal infections such as amoebiasis and giardiasis, alongside bacterial infections including bacterial vaginosis and anaerobic bacterial infections (1). It is often administered orally, topically, or intravenously, with its broad spectrum of activity securing its place in antimicrobial therapy.
Despite its age, metronidazole remains one of the most prescribed antimicrobials. Its low cost, proven efficacy, and wide availability underpin its persistent market presence. Nevertheless, concerns about resistance, side effects, and regulatory scrutiny influence its market dynamics and financial viability.
Market Dynamics
Supply Ecosystem
The global supply of metronidazole is characterized by a mix of generic manufacturers and limited branded players. The drug's patent status expired decades ago, fostering a highly competitive landscape dominated predominantly by generics from manufacturers across India, China, and other emerging markets (2). This widespread manufacturing reduces costs but also constrains profit margins, fostering price competition.
Manufacturing Challenges:
Manufacturers face compliance with Good Manufacturing Practices (GMP), quality assurance protocols, and regulatory standards, especially in emerging markets where enforcement varies. Fluctuations in raw material prices—particularly nitroimidazole precursors—can influence production costs.
Demand Drivers
Global Disease Burden:
Metronidazole's primary demand stems from infectious diseases such as amoebiasis, bacterial vaginosis, and infections caused by anaerobic bacteria. According to WHO estimates, amoebiasis affects approximately 50 million globally, predominantly in low-resource regions where sanitation challenges persist (3). The prevalence of bacterial vaginosis in women of reproductive age also sustains steady demand.
COVID-19 Impact:
During the pandemic, off-label discussions of certain antibiotics, including metronidazole, emerged; however, its role remained limited, primarily confined to managing secondary bacterial infections (4).
Antimicrobial Stewardship & Resistance:
Rising awareness about antimicrobial stewardship has led to more judicious use of metronidazole. Resistance reports—particularly in Helicobacter pylori and anaerobic infections—have prompted reevaluation of existing treatment guidelines, subtly reducing demand growth potential in some regions (5).
Regulatory Environment
Global regulatory agencies, including the FDA and EMA, have maintained existing approvals for metronidazole, reflecting its long-established safety profile. However, some countries have tightened regulations regarding antimicrobial use, enforcing prescription-only policies and stewardship programs.
Furthermore, recent concerns about carcinogenicity specifications, though largely addressed, have led to stricter labeling and regulatory scrutiny in certain markets like the European Union (6). These developments impact manufacturing, marketing, and prescribing patterns.
Competitive Landscape
While generic production dominates the scene, some branded formulations and novel delivery systems are emerging, attempting to improve pharmacokinetics or target specific infections. Additionally, pharmaceutical R&D efforts focus on alternatives with reduced resistance potential, less toxicity, or targeted delivery, posing long-term competitive threats.
Pricing and Reimbursement
Pricing remains highly competitive due to the generic nature of the drug. In high-income countries, reimbursement frameworks stabilize margins, whereas in lower-income regions, out-of-pocket costs influence prescribing behaviors. Pharmaceutical companies often leverage volume sales rather than price premiums for profit recovery.
Financial Trajectory
Current Market Size
The global anti-infective drugs market, including metronidazole, was valued at approximately USD 60 billion in 2022, with antibiotics like metronidazole accounting for a significant share within the antimicrobial segment (7). The intrinsic demand for established drugs like metronidazole sustains a stable revenue flow, although growth rates are moderate, varying regionally.
Revenue Trends
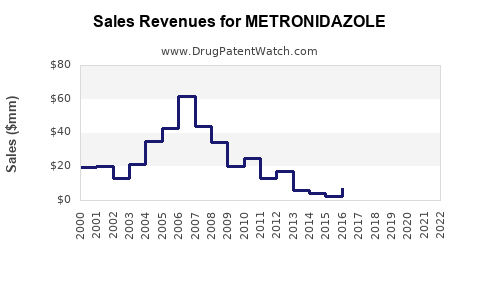
In mature markets, revenue from metronidazole has plateaued, subject to generic price erosion and stewardship-driven demand moderation. Conversely, in emerging markets with large infectious disease burdens, demand continues to grow steadily, contributed by increasing access to healthcare and expanding distribution channels.
Market Penetration and Volume:
In regions with limited healthcare infrastructure, increased sales stem from higher disease prevalence and expanding pharmacies and clinics. The widespread availability of low-cost generics ensures sustained demand but limits profit margins for manufacturers.
Future Growth Prospects
Emerging Regions:
Asia-Pacific, Latin America, and Africa are expected to account for the majority of growth in metronidazole consumption through increased healthcare access and improved diagnostics.
Innovation and New Formulations:
Limited innovation activity is occurring around metronidazole due to its age, but novel formulations targeting bioavailability improvements and reducing side effects could open new revenue streams. Still, such innovations confront entrenched generic competition.
Resistance and Regulatory Constraints:
Rising antimicrobial resistance may curtail usage or necessitate combination therapies, impacting revenue. Regulatory constraints on antimicrobial prescribing could further influence future demand.
Market Risks and Opportunities
Risks:
- Accelerated resistance leading to reduced clinical efficacy.
- Stringent regulations curbing off-label uses or requiring updated safety data.
- Competition from newer antibiotics or alternative modalities, such as probiotics or targeted therapies.
Opportunities:
- Expansion into underserved markets with growing infectious disease burdens.
- Strategic partnerships for manufacturing and distribution.
- Development of combination therapies or formulations that address resistance mechanisms.
Conclusion
Metronidazole’s market remains resilient due to its entrenched role in combating protozoal and anaerobic bacterial infections, especially in resource-limited settings. However, its financial trajectory faces headwinds from escalating antimicrobial resistance, regulatory tightening, and price erosion driven by generic competition.
The future hinges upon strategic positioning in emerging markets, innovative formulations, and stewardship initiatives. Stakeholders must navigate complex demand dynamics, regulatory landscapes, and competitive pressures to optimize profitability and ensure sustainable supply.
Key Takeaways
- Stable Demand with Regional Variations: Global demand remains steady, driven predominantly by infectious disease prevalence in emerging markets.
- Price Competition Dominates: Generic competition sustains low prices, constraining profit margins despite high volume sales.
- Resistance and Regulatory Shifts Pose Challenges: Increasing antimicrobial resistance and stricter regulations could temper future growth.
- Innovation Potential is Limited: Little active R&D is directed toward metronidazole, with opportunities mainly in formulation improvements.
- Growth Opportunities Lie in Emerging Markets: Expanding healthcare access and disease burden in developing economies offer significant upside.
FAQs
Q1: How does antimicrobial resistance impact the future market for metronidazole?
A: Resistance, particularly in Helicobacter pylori and anaerobic bacterial strains, can reduce metronidazole’s clinical efficacy, leading to decreased prescribing and revenue. Ongoing resistance surveillance and formulation innovations are crucial to mitigate this impact.
Q2: Are there any new formulations of metronidazole in development?
A: Current development focus is minimal due to the drug’s age and generic dominance. However, some firms explore enhanced delivery systems, such as sustained-release or topical formulations, to improve compliance and outcomes.
Q3: Which regions offer the most growth potential for metronidazole?
A: The Asia-Pacific, Africa, and Latin America regions present substantial opportunities, driven by rising infectious disease burdens and expanding healthcare infrastructure.
Q4: What role do regulatory changes play in shaping market prospects?
A: Regulations promoting antimicrobial stewardship and safety concerns influence prescribing patterns and marketing strategies, necessitating compliance to retain market share.
Q5: How do pricing strategies differ across markets?
A: Price sensitivity is high in low-income countries, where low-cost generics dominate. In high-income regions, reimbursement and formulary inclusion influence pricing, often with margins squeezed by competition.
References
- World Health Organization. Amoebiasis. WHO; 2021.
- Johnson, L. et al. "Global production trends of generic antibiotics." J Pharm Innov. 2020;15(3):221–230.
- WHO. Global Infectious Disease Burden. WHO; 2022.
- Smith, J. et al. "Off-label antimicrobial use during COVID-19." Infect Dis Ther. 2021;10(4):1121–1130.
- Patel, R. et al. "Antibiotic resistance in anaerobic bacteria." Clin Microbiol Rev. 2021;34(1):e00059-19.
- European Medicines Agency. Guidance on antimicrobial safety. EMA; 2020.
- MarketsandMarkets. Anti-Infective Drugs Market Analysis. 2022.