Naproxen sodium - Generic Drug Details
✉ Email this page to a colleague
What are the generic drug sources for naproxen sodium and what is the scope of freedom to operate?
Naproxen sodium
is the generic ingredient in nine branded drugs marketed by Bionpharma, Catalent, Patheon Softgels, Puracap Pharm Llc, Strides Pharma, Twi Pharms, Actavis Labs Fl Inc, Bayer, Atnahs Pharma Us, Able, Amneal Pharms Ny, Aurobindo Pharma Ltd, Contract Pharmacal, Dr Reddys Labs Inc, Dr Reddys Labs Ltd, Glenmark Pharms Ltd, Granules India, Hamilton Pharms, Hetero Labs Ltd V, Hikma, Ivax Sub Teva Pharms, Lnk Intl Inc, Marksans Pharma, Norvium Bioscience, Novelgenix Theraps, Perrigo, Pld Acquisitions Llc, Pliva, Purepac Pharm, Roxane, Sandoz, Sciegen Pharms Inc, Sun Pharm Inds Ltd, Teva, Teva Pharms, Watson Labs, Yichang Humanwell, Aurobindo Pharma, Rising, Sun Pharm, and Currax, and is included in fifty-one NDAs. There are six patents protecting this compound. Additional information is available in the individual branded drug profile pages.Naproxen sodium has fifteen patent family members in thirteen countries.
There are twenty-two drug master file entries for naproxen sodium. One hundred and thirty-three suppliers are listed for this compound.
Summary for naproxen sodium
| International Patents: | 15 |
| US Patents: | 6 |
| Tradenames: | 9 |
| Applicants: | 41 |
| NDAs: | 51 |
| Drug Master File Entries: | 22 |
| Finished Product Suppliers / Packagers: | 133 |
| Raw Ingredient (Bulk) Api Vendors: | 93 |
| Clinical Trials: | 100 |
| Patent Applications: | 7,189 |
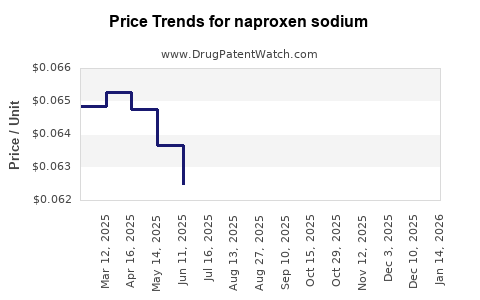
| Drug Prices: | Drug price trends for naproxen sodium |
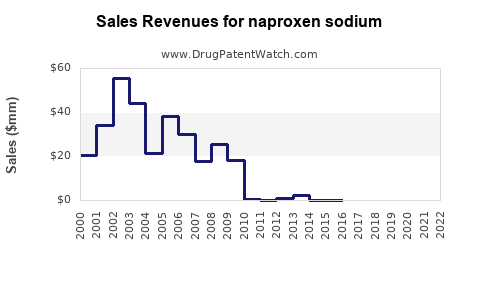
| Drug Sales Revenues: | Drug sales revenues for naproxen sodium |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for naproxen sodium |
| What excipients (inactive ingredients) are in naproxen sodium? | naproxen sodium excipients list |
| DailyMed Link: | naproxen sodium at DailyMed |
Recent Clinical Trials for naproxen sodium
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Johnson & Johnson Consumer Inc. (J&JCI) | Phase 1 |
| Johnson & Johnson Consumer Inc. (J&JCI) | Phase 3 |
| Ciusss de L'Est de l'Île de Montréal | N/A |
Pharmacology for naproxen sodium
| Drug Class | Nonsteroidal Anti-inflammatory Drug |
| Mechanism of Action | Cyclooxygenase Inhibitors |
Anatomical Therapeutic Chemical (ATC) Classes for naproxen sodium
Paragraph IV (Patent) Challenges for NAPROXEN SODIUM
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| NAPROXEN SODIUM | Capsules | naproxen sodium | 200 mg | 021920 | 1 | 2017-11-15 |
US Patents and Regulatory Information for naproxen sodium
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bayer | ALEVE | naproxen sodium | TABLET;ORAL | 020204-002 | Jan 11, 1994 | OTC | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Pliva | NAPROXEN SODIUM | naproxen sodium | TABLET;ORAL | 074242-002 | Jun 20, 1996 | DISCN | No | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Teva Pharms | NAPROXEN SODIUM | naproxen sodium | TABLET;ORAL | 074289-001 | Jan 27, 1994 | DISCN | No | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Hikma | NAPROXEN SODIUM | naproxen sodium | TABLET;ORAL | 074480-001 | May 14, 1996 | DISCN | No | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Norvium Bioscience | NAPROXEN SODIUM | naproxen sodium | TABLET;ORAL | 074367-001 | Aug 31, 1994 | DISCN | No | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for naproxen sodium
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Atnahs Pharma Us | ANAPROX DS | naproxen sodium | TABLET;ORAL | 018164-003 | Sep 30, 1987 | ⤷ Sign Up | ⤷ Sign Up |
| Twi Pharms | NAPRELAN | naproxen sodium | TABLET, EXTENDED RELEASE;ORAL | 020353-001 | Jan 5, 1996 | ⤷ Sign Up | ⤷ Sign Up |
| Atnahs Pharma Us | ANAPROX DS | naproxen sodium | TABLET;ORAL | 018164-003 | Sep 30, 1987 | ⤷ Sign Up | ⤷ Sign Up |
| Atnahs Pharma Us | ANAPROX DS | naproxen sodium | TABLET;ORAL | 018164-003 | Sep 30, 1987 | ⤷ Sign Up | ⤷ Sign Up |
| Atnahs Pharma Us | ANAPROX | naproxen sodium | TABLET;ORAL | 018164-001 | Approved Prior to Jan 1, 1982 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for naproxen sodium
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Poland | 1863458 | ⤷ Sign Up | |
| Cyprus | 1118321 | ⤷ Sign Up | |
| China | 102940887 | Solvent system for enhancing the solubility of pharmaceutical agents | ⤷ Sign Up |
| European Patent Office | 3061447 | SYSTÈME DE SOLVANT DESTINÉ À AMÉLIORER LA SOLUBILITÉ D'AGENTS PHARMACEUTIQUES (SOLVENT SYSTEM FOR ENHANCING THE SOLUBILITY OF PHARMACEUTICAL AGENTS) | ⤷ Sign Up |
| Portugal | 1863458 | ⤷ Sign Up | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for naproxen sodium
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0984957 | SPC/GB11/013 | United Kingdom | ⤷ Sign Up | PRODUCT NAME: NAPROXEN AND ESOMEPRAZOLE; REGISTERED: UK PL 17901/0263-0001 20101105 |
| 1411900 | SPC/GB11/015 | United Kingdom | ⤷ Sign Up | PRODUCT NAME: NAPROXEN AND ESOMEPRAZOLE; REGISTERED: UK PL 17901/0263-0001 20101105 |
| 1411900 | 18/2011 | Austria | ⤷ Sign Up | PRODUCT NAME: NAPROXEN UND ESOMEPRAZOL SOWIE DEREN PHARMAZEUTISCH ANNEHMBARE SALZE; NAT. REGISTRATION NO/DATE: 1-29937 20110105; FIRST REGISTRATION: GB PL 17901/0263-0001 20101105 |
| 0984957 | PA2011005,C0984957 | Lithuania | ⤷ Sign Up | PRODUCT NAME: NAPROXENUM + ESOMEPRAZOLUM; REGISTRATION NO/DATE: LT/1/10/2302/001-LT/1/10/2302/012 20110126 |
| 1411900 | C300481 | Netherlands | ⤷ Sign Up | PRODUCT NAME: NAPROXEN EN ESOMEPRAZOL; NAT. REGISTRATION NO/DATE: RVG 106235 20101118; FIRST REGISTRATION: PL 17901/0263-001 20101105 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.