Last updated: September 24, 2025
Introduction
Budesonide, a potent corticosteroid primarily used to treat inflammatory and allergic conditions, plays a significant role in respiratory, gastrointestinal, and ENT therapeutic areas. Since its emergence, the drug has evolved within a competitive landscape dominated by a combination of branded formulations and generics. Its market dynamics are shaped by patent expiries, regulatory pathways, clinical innovations, and global health trends, influencing its financial trajectory across therapeutic segments.
Pharmacological Profile and Therapeutic Applications
Budesonide is distinguished by its high topical potency and extensive first-pass metabolism, which minimizes systemic side effects. It is approved across various formulations, including oral, inhalational, nasal, and rectal preparations, each catering to specific indications:
- Inhalation (Pulmonary): Used for asthma and Chronic Obstructive Pulmonary Disease (COPD).
- Nasal: Approved for allergic rhinitis and nasal polyposis.
- Oral: Employed in Crohn’s disease and ulcerative colitis.
- Rectal: Utilized for ulcerative proctitis.
This versatility broadens its revenue streams, underpinning its sustainable market presence.
Market Dynamics
1. Patent and Regulatory Landscape
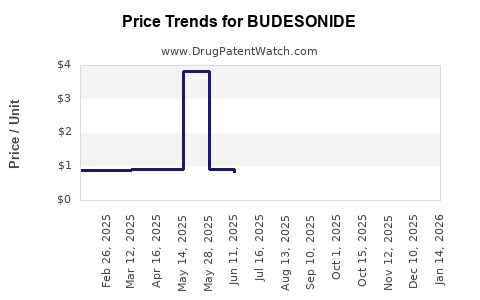
Budesonide’s initial formulations, such as Pulmicort (for asthma), benefited from patent protections that drove early market penetration. Typically, patent exclusivity spans 10-15 years, after which generics enter the marketplace.
The expiration of key patents—particularly in the U.S. and Europe—has catalyzed the proliferation of generic versions, intensifying price competition, reducing costs, and increasing accessibility. Regulatory agencies have also fast-tracked approvals of new formulations and delivery systems, further shaping market dynamics.
2. Competitive Environment
The market features a mixture of brand-name drugs like Pulmicort (AstraZeneca) and Rhinocort (AstraZeneca), alongside numerous generic manufacturers. The introduction of biosimilars and alternative corticosteroids exerts downward pressure on prices and margins.
Innovative delivery systems, such as hydrofluoroalkane (HFA) inhalers, have enhanced drug efficacy and patient adherence, asserting competitive differentiation.
3. Clinical and Technological Innovations
Recent advancements include biodegradable nasal sprays and dual-action inhalers that combine budesonide with long-acting bronchodilators. These innovations aim to improve efficacy, reduce dosing frequency, and foster patient compliance—potentially capturing new market segments and driving revenue.
4. Geographic and Demographic Factors
Growth in emerging markets like China, India, and Latin America presents significant opportunities due to rising respiratory and allergic disease prevalence amid increasing healthcare access. Developed markets, meanwhile, are characterized by a maturation phase with stable or declining growth, influenced by patent expiry and generic penetration.
5. Impact of COVID-19 and Pandemic-Driven Trends
While not directly aligned with COVID-19 treatments, budesonide’s role in managing respiratory symptoms and its use in off-label settings boosted awareness and demand during the pandemic. This temporarily stabilized sales dynamics and highlighted the importance of corticosteroids in respiratory care.
Financial Trajectory and Revenue Outlook
1. Revenue Drivers
- Brand loyalty and clinical efficacy: Sustains premium pricing in branded segments.
- Generic competition: Deters sustained price hikes, but volume increases often compensate, especially in emerging markets.
- Innovative delivery systems: Premium priced, functionally differentiated products bolster margins and revenue.
2. Market Size and Forecast
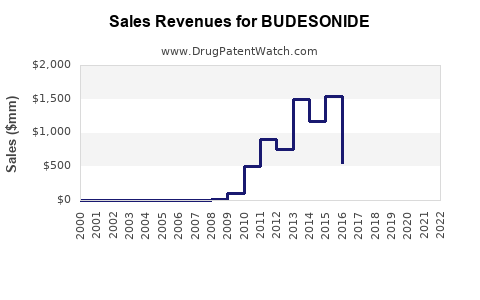
The global inhaled corticosteroids market was valued at approximately USD 5 billion in 2022 and is projected to grow at a CAGR of 4.5% through 2030, driven by expanding asthma and COPD patient populations [1]. The nasal corticosteroids segment, including budesonide-based products, is expected to similarly expand, reaching USD 3.2 billion by 2030.
In the gastrointestinal segment, increasing indications for budesonide suggest a compound annual growth rate (CAGR) of roughly 5%, driven by unmet needs and ongoing clinical research. The combination of stable developed markets and high-growth emerging economies sustains the overall positive financial trajectory.
3. Key Revenue Influencers
- Patent expiries: Anticipated expirations in North America and Europe within the next 3–5 years could lead to revenue declines unless offset by volume growth or incremental innovations.
- Regulatory approvals: New formulations and combination therapies may unlock premium pricing and extended market share.
- Market penetration: Entry into new markets and increased prescription adherence through improved formulations will be pivotal.
4. Investment and R&D Impact
Continued investment in inhaler technology, nasal spray devices, and oral formulations positions companies to maintain competitive advantages. R&D efforts also focus on indications like eosinophilic esophagitis and systemic inflammatory conditions, potentially expanding the addressable market.
Challenges and Opportunities
Challenges
- Generic erosion: Patent expiries significantly impact revenue streams.
- Pricing pressures: Healthcare reimbursement policies prioritize cost-efficiency, especially in developed markets.
- Competitive landscape: Emergence of new corticosteroids and biologics for inflammatory diseases threaten market share.
Opportunities
- Innovation pipeline: Novel delivery systems and combination therapies.
- Global health trends: Rising prevalence of asthma, COPD, and allergic diseases in developing countries.
- Regulatory strategies: Fast-track approvals and patent extensions through new indications and formulations.
Regulatory and Patent Outlook
Regulatory authorities are increasingly allowing flexible approval pathways for new formulations and delivery devices, which can extend product lifecycles. Meanwhile, companies seek to preserve market exclusivity via patent strategies, formulation patents, and data exclusivity periods.
Conclusion
Budesonide's market dynamics are characterized by a cyclical transition from patent-protected products to widespread generic availability, punctuated by continual innovation and geographic expansion. While patent expiries pose revenue risks, leveraging formulation innovation, expanding into emerging markets, and addressing unmet needs sustain its financial trajectory. The future of budesonide hinges on strategic R&D investments, regulatory agility, and market positioning within the competitive corticosteroid landscape.
Key Takeaways
- Patent expiries and generics dominate near-term market challenges but are offset by innovations and expanding markets.
- Emerging markets offer high-growth opportunities driven by increasing respiratory and allergic disease prevalence.
- Formulation and delivery system innovations are critical for maintaining premium pricing and patient adherence.
- Regulatory pathways and patent strategies play pivotal roles in extending product lifecycle and revenue stability.
- Continued investment in clinical research can unlock new indications and therapeutic combinations, diversifying revenue streams.
FAQs
1. How does patent expiration affect budesonide’s market share?
Patent expirations typically lead to increased generic competition, reducing prices and margins for branded formulations. However, introduced innovations and newer delivery mechanisms can offset revenue loss by attracting different patient segments and maintaining brand loyalty.
2. What are the key therapeutic segments for budesonide?
Budesonide is widely used in respiratory (asthma, COPD), nasal (allergic rhinitis, polyposis), and gastrointestinal (Crohn’s disease, ulcerative colitis) indications.
3. Which emerging markets present the greatest growth potential for budesonide?
China, India, and Latin America are expanding rapidly due to increasing disease prevalence, urbanization, and improving healthcare infrastructure.
4. Are there upcoming regulatory hurdles for budesonide?
Regulatory challenges include approval of new formulations, biosimilars, and navigating pricing and reimbursement policies, especially in heavily regulated markets like the U.S. and Europe.
5. How does innovation influence budesonide’s long-term market viability?
Innovations in inhaler technology, nasal spray formulations, and combination therapies are vital to differentiating products, extending patent life, and delivering sustained revenue streams.
Sources
[1] Grand View Research. "Inhaled Corticosteroids Market Size & Trends Analysis." 2022.