ATORVASTATIN CALCIUM - Generic Drug Details
✉ Email this page to a colleague
What are the generic sources for atorvastatin calcium and what is the scope of freedom to operate?
Atorvastatin calcium
is the generic ingredient in five branded drugs marketed by Cmp Dev Llc, Accord Hlthcare, Alkem Labs Ltd, Apotex, Apotex Inc, Aurobindo Pharma Ltd, Biocon Pharma, Cadila Pharms Ltd, Chartwell Rx, Dr Reddys, Dr Reddys Labs Ltd, Graviti Pharms, Hetero Labs Ltd V, Invagen Pharms, Lannett Co Inc, Laurus, Lepu Pharm, Lupin Ltd, Macleods Pharms Ltd, Mankind Pharma, Micro Labs Ltd India, MSN, Mylan Pharms Inc, Perrigo R And D, Sciegen Pharms Inc, Shandong Xinhua, Strides Pharma, Sun Pharm Inds Ltd, Teva Pharms, Teva Pharms Inc, Teva Pharms Usa, Umedica, Zydus Pharms, Upjohn, Organon, and Pharmobedient, and is included in thirty-seven NDAs. There are five patents protecting this compound. Additional information is available in the individual branded drug profile pages.Atorvastatin calcium has two patent family members in two countries.
There are fifty-five drug master file entries for atorvastatin calcium. Sixty suppliers are listed for this compound.
Summary for ATORVASTATIN CALCIUM
| International Patents: | 2 |
| US Patents: | 5 |
| Tradenames: | 5 |
| Applicants: | 36 |
| NDAs: | 37 |
| Drug Master File Entries: | 55 |
| Finished Product Suppliers / Packagers: | 60 |
| Raw Ingredient (Bulk) Api Vendors: | 1 |
| Clinical Trials: | 72 |
| Patent Applications: | 6,089 |
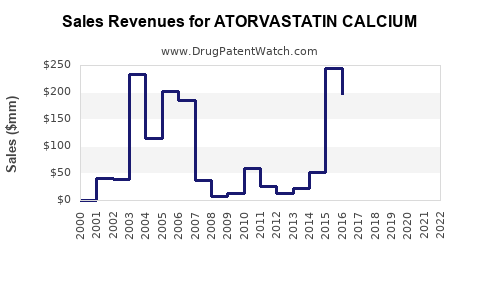
| Drug Sales Revenues: | Drug sales revenues for ATORVASTATIN CALCIUM |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for ATORVASTATIN CALCIUM |
| What excipients (inactive ingredients) are in ATORVASTATIN CALCIUM? | ATORVASTATIN CALCIUM excipients list |
| DailyMed Link: | ATORVASTATIN CALCIUM at DailyMed |
Recent Clinical Trials for ATORVASTATIN CALCIUM
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| NewAmsterdam Pharma | Phase 1 |
| Pharma Medica Research, Inc. | Phase 1 |
| University of California, San Francisco | Phase 2 |
Pharmacology for ATORVASTATIN CALCIUM
| Drug Class | HMG-CoA Reductase Inhibitor |
| Mechanism of Action | Hydroxymethylglutaryl-CoA Reductase Inhibitors |
Medical Subject Heading (MeSH) Categories for ATORVASTATIN CALCIUM
Anatomical Therapeutic Chemical (ATC) Classes for ATORVASTATIN CALCIUM
US Patents and Regulatory Information for ATORVASTATIN CALCIUM
Expired US Patents for ATORVASTATIN CALCIUM
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Upjohn | LIPITOR | atorvastatin calcium | TABLET;ORAL | 020702-004 | Apr 7, 2000 | ⤷ Get Started Free | ⤷ Get Started Free |
| Upjohn | LIPITOR | atorvastatin calcium | TABLET;ORAL | 020702-002 | Dec 17, 1996 | ⤷ Get Started Free | ⤷ Get Started Free |
| Upjohn | LIPITOR | atorvastatin calcium | TABLET;ORAL | 020702-003 | Dec 17, 1996 | ⤷ Get Started Free | ⤷ Get Started Free |
| Upjohn | LIPITOR | atorvastatin calcium | TABLET;ORAL | 020702-004 | Apr 7, 2000 | ⤷ Get Started Free | ⤷ Get Started Free |
| Upjohn | LIPITOR | atorvastatin calcium | TABLET;ORAL | 020702-004 | Apr 7, 2000 | ⤷ Get Started Free | ⤷ Get Started Free |
| Upjohn | LIPITOR | atorvastatin calcium | TABLET;ORAL | 020702-002 | Dec 17, 1996 | ⤷ Get Started Free | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for ATORVASTATIN CALCIUM
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| European Patent Office | 3468606 | NOUVELLE COMPOSITION PHARMACEUTIQUE DE COMPOSÉ HYPOLIPIDÉMIANT (A NOVEL PHARMACEUTICAL COMPOSITION OF A LIPID LOWERING COMPOUND) | ⤷ Get Started Free |
| World Intellectual Property Organization (WIPO) | 2017212409 | ⤷ Get Started Free | |
| European Patent Office | 3468606 | ⤷ Get Started Free | |
| World Intellectual Property Organization (WIPO) | 2017212409 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for ATORVASTATIN CALCIUM
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0720599 | 122014000088 | Germany | ⤷ Get Started Free | PRODUCT NAME: EZETIMIB ODER PHARMAZEUTISCH ANNEHMBARE SALZE DAVON IN KOMBINATION MIT ATORVASTATIN ODER PHARMAZEUTISCH ANNEHMBARE SALZE DAVON, INSBESONDERE ATORVASTATIN ALS ATORVASTATINCALCIUMTRIHYDRAT; NAT. REGISTRATION NO/DATE: 90223.00.00 20141113; FIRST REGISTRATION: FRANKREICH CIS: 6 928 917 6 20140912 |
| 1003503 | 30/2006 | Austria | ⤷ Get Started Free | PRODUCT NAME: AMLODIPIN ODER EIN PHARMAZEUTISCH ANNEHMBARES SAEUREADDITIONSALZ DESSELBEN IN KOMBINATION MIT ATORVASTATIN ODER EINEM PHARMAZEUTISCH ANNEHMBAREN SALZ DESSELBEN; NAT. REGISTRATION NO/DATE: 1-26271, 1-26273 20060330; FIRST REGISTRATION: FR 369300.9;369301.5; 369302.1;369303.8;369304.4;369305.0; 20050707 |
| 0247633 | C970034 | Netherlands | ⤷ Get Started Free | PRODUCT NAME: ATORVASTATINUM,DESGEWENST IN DE VORM VAN EEN FARMACEUTISCH AANVAARDBAAR ZOUT OF VAN HET INWENDIGE DELTA-LACTON, IN HET BIJZONDER ATORVASTATINUM CALCICUM TRIHYDRICUM; NAT. REGISTRATION NO/DATE: RVG 21081 - RVG 21083 19970421; FIRST REGISTRATION: GB PL 00018/0240 - PL 00018/0242 19961107 |
| 0720599 | CR 2014 00050 | Denmark | ⤷ Get Started Free | PRODUCT NAME: EZETIMIBE AND ATORVASTATIN OR PHARMACEUTICALLY ACCEPTABLE SALTS THEREOF, INCLUDING ATORVASTATIN AS ATORVASTATIN CALCIUM TRIHYDRATE; REG. NO/DATE: DE/H/3895-3898/001-004/DC 20140910 |
| 1003503 | C01003503/01 | Switzerland | ⤷ Get Started Free | PRODUCT NAME: AMLODIPIN + ATORVASTATIN; REGISTRATION NUMBER/DATE: SWISSMEDIC 57633 05.07.2006 |
| 0247633 | 97C0103 | Belgium | ⤷ Get Started Free | PRODUCT NAME: CALCII ATORVASTATINUM TRIHYDRICUM (=ATORVASTATINUM); NAT. REGISTRATION NO/DATE: 19 IS 95 F 3 19970922; FIRST REGISTRATION: GB PL/00018/0240 19961107 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for the Pharmaceutical Drug: Atorvastatin Calcium
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.