MSD SUB MERCK Company Profile
✉ Email this page to a colleague
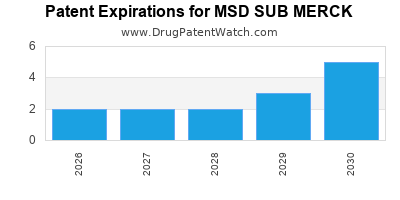
What is the competitive landscape for MSD SUB MERCK, and when can generic versions of MSD SUB MERCK drugs launch?
MSD SUB MERCK has fourteen approved drugs.
There are twenty US patents protecting MSD SUB MERCK drugs.
There are five hundred and forty-nine patent family members on MSD SUB MERCK drugs in sixty countries and one hundred and fifty-eight supplementary protection certificates in nineteen countries.
Summary for MSD SUB MERCK
| International Patents: | 549 |
| US Patents: | 20 |
| Tradenames: | 13 |
| Ingredients: | 11 |
| NDAs: | 14 |
Drugs and US Patents for MSD SUB MERCK
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Msd Sub Merck | BRIDION | sugammadex sodium | SOLUTION;INTRAVENOUS | 022225-002 | Dec 15, 2015 | AP | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ||||
| Msd Sub Merck | SEGLUROMET | ertugliflozin; metformin hydrochloride | TABLET;ORAL | 209806-003 | Dec 19, 2017 | RX | Yes | No | 9,308,204 | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Msd Sub Merck | BRIDION | sugammadex sodium | SOLUTION;INTRAVENOUS | 022225-001 | Dec 15, 2015 | AP | RX | Yes | Yes | RE44733 | ⤷ Sign Up | Y | Y | ⤷ Sign Up | |
| Msd Sub Merck | SEGLUROMET | ertugliflozin; metformin hydrochloride | TABLET;ORAL | 209806-004 | Dec 19, 2017 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | |||||
| Msd Sub Merck | SEGLUROMET | ertugliflozin; metformin hydrochloride | TABLET;ORAL | 209806-003 | Dec 19, 2017 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for MSD SUB MERCK
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Msd Sub Merck | JANUMET XR | metformin hydrochloride; sitagliptin phosphate | TABLET, EXTENDED RELEASE;ORAL | 202270-002 | Feb 2, 2012 | 6,635,280 | ⤷ Sign Up |
| Msd Sub Merck | INVANZ | ertapenem sodium | INJECTABLE;INTRAMUSCULAR, INTRAVENOUS | 021337-001 | Nov 21, 2001 | 5,952,323*PED | ⤷ Sign Up |
| Msd Sub Merck | BRIDION | sugammadex sodium | SOLUTION;INTRAVENOUS | 022225-001 | Dec 15, 2015 | 6,949,527 | ⤷ Sign Up |
| Msd Sub Merck | JANUMET | metformin hydrochloride; sitagliptin phosphate | TABLET;ORAL | 022044-001 | Mar 30, 2007 | 7,459,428 | ⤷ Sign Up |
| Msd Sub Merck | ZOLINZA | vorinostat | CAPSULE;ORAL | 021991-001 | Oct 6, 2006 | 8,101,663 | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for MSD SUB MERCK drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Extended-release Tablets | 100 mg/1000 mg | ➤ Subscribe | 2012-10-22 |
| ➤ Subscribe | Injection | 2 mg/mL, 10 mL vial | ➤ Subscribe | 2008-09-30 |
| ➤ Subscribe | Injection | 0.75 mg/mL, 100 mL vial | ➤ Subscribe | 2009-06-05 |
| ➤ Subscribe | Tablets | 400 mg | ➤ Subscribe | 2011-10-12 |
| ➤ Subscribe | Extended-release Capsules | 50 mg/500 mg and 50 mg/1000 mg | ➤ Subscribe | 2012-03-16 |
| ➤ Subscribe | Tablets | 50 mg/500 mg and 50 mg/1000 mg | ➤ Subscribe | 2010-10-18 |
| ➤ Subscribe | Injection | 2 mg/mL, 100 mL vial | ➤ Subscribe | 2008-12-18 |
| ➤ Subscribe | Injection | 1 g/vial | ➤ Subscribe | 2013-06-20 |
| ➤ Subscribe | Ophthalmic Solution | 2% | ➤ Subscribe | 2005-10-11 |
International Patents for MSD SUB MERCK Drugs
Supplementary Protection Certificates for MSD SUB MERCK Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2498758 | PA2020003,C2498758 | Lithuania | ⤷ Sign Up | PRODUCT NAME: METFORMINO HIDROCHLORIDAS; SAKSAGLIPTINAS ARBA FARMACINIU POZIURIU PRIIMTINA JO DRUSKA; DAPAGLIFLOZINAS ARBA FARMACINIU POZIURIU PRIIMTINAS JO SOLVATAS; REGISTRATION NO/DATE: EU/1/19/1401 20191111 |
| 2498758 | CA 2020 00017 | Denmark | ⤷ Sign Up | PRODUCT NAME: METFORMIN ELLER ET FARMACEUTISK ACCEPTABELT SALT DERAF; SAXAGLIPTIN ELLER ET FARMACEUTISK ACCEPTABELT SALT DERAF; DAPAGLIFLOZIN ELLER ET FARMACEUTISK ACCEPTABELT SOLVAT DERAF; REG. NO/DATE: EU/1/19/1401 20191113 |
| 1441735 | PA2008007,C1441735 | Lithuania | ⤷ Sign Up | PRODUCT NAME: RALTEGRAVIRUM; REGISTRATION NO/DATE: EU/1/07/436/001, 2007 12 20 EU/1/07/436/002 20071220 |
| 1441735 | 0890018-5 | Sweden | ⤷ Sign Up | PRODUCT NAME: RALTEGRAVIR |
| 2334687 | PA2018510 | Lithuania | ⤷ Sign Up | PRODUCT NAME: ERTUGLIFLOZINAS, PASIRINKTINAI KAIP KRISTALINE FORMA, YPAC KAIP KO-KRISTALAS SU L-PIROGLUTAMO RUGSTIMI, IR YPAC KAIP ERTUGLIFLOZINO L-PIROGLUTAMO RUGSTIS; REGISTRATION NO/DATE: EU/1/18/1267 20180321 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.