Last updated: July 29, 2025
Introduction
MSD Merck & Co., commonly known as Merck in the U.S. and Canada, is a global leader in pharmaceutical innovation with a rich history spanning over a century. Its expansive portfolio includes vaccines, oncology, infectious diseases, cardiovascular, and endocrinology products. As healthcare landscapes evolve rapidly driven by technological advances and regulatory challenges, understanding Merck’s market position, strengths, and strategic direction provides critical insights for stakeholders navigating the pharmaceutical industry.
Market Position of MSD Merck
Merck maintains a formidable market presence, consistently ranking among the top-tier pharmaceutical companies worldwide. According to IMS Health data, Merck achieved approximately $59 billion in global revenues in 2022, positioning it among the top five global pharma players. Its diversified product portfolio covers several lucrative therapeutic areas, notably oncology with blockbuster drugs like Keytruda (pembrolizumab), vaccines such as Gardasil, and infectious disease treatments like Januvia (sitagliptin).
Key markets span North America, Europe, Asia-Pacific, and emerging economies, underpinned by a robust pipeline of novel therapeutics and vaccine technologies. Merck's substantial investments in R&D—over $10 billion annually—fortify its innovation pipeline and sustain its competitive edge.
Furthermore, Merck's strategic focus on collaborations and acquisitions, such as the 2019 acquisition of Peloton Therapeutics and the partnership with Moderna for mRNA vaccine development, enhances its market agility. Its leadership in oncology, vaccine development, and immunotherapy affirms its position as a market innovator and authority.
Core Strengths of MSD Merck
1. Dominance in Oncology and Immunotherapy
Merck’s flagship drug, Keytruda, revolutionized cancer treatment by expanding indications across multiple tumor types, including melanoma, lung, bladder, and gastric cancers. It generated over $20 billion in sales in 2022, establishing Keytruda as the highest-selling immunotherapy globally. Its broad approval pipeline and continual label expansions amplify long-term revenue prospects.
2. Leadership in Vaccines
Merck remains a leader in vaccine development, especially in HPV (Gardasil), shingles (Shingrix), and pneumococcal vaccines. The company’s vaccine portfolio accounts for approximately 20-25% of its global revenue and benefits from high market penetration, especially in developed economies.
3. Robust R&D Ecosystem
Investing heavily in R&D, Merck maintains a pipeline of over 75 late-stage candidates and strategic collaborations in gene therapy, mRNA, and personalized medicine, driving future growth.
4. Strong Global Presence
With operations in over 140 countries, Merck's extensive distribution network enhances market access, particularly in emerging markets. Its established manufacturing capabilities and strategic alliances support rapid product commercialization.
5. Strategic Alliances and Acquisitions
Collaborations with Moderna for mRNA vaccine development and investments in emerging biotech firms strengthen Merck’s innovation ecosystem and diversify revenue streams. These partnerships enable rapid adaptation to global health challenges and capitalize on cutting-edge science.
Strategic Insights and Opportunities
1. Expansion in Oncology and Immunotherapy
Although Keytruda’s dominance is challenged by emerging competitors, continuous label extensions and combination therapies amplify its lifecycle value. Merck should prioritize biomarker-driven clinical trials and real-world evidence to maintain leadership and prevent market erosion.
2. mRNA and Vaccine Innovation
The COVID-19 pandemic highlighted the transformative potential of mRNA technology. Merck’s partnership with Moderna positions it to refine vaccine platforms, expand into personalized vaccines, and explore mRNA therapies for other diseases, including cancer and rare infections.
3. Focus on Precision Medicine
Investing in biomarker development and companion diagnostics enhances targeted therapies' efficacy. Strengthening collaborations in genomics and AI-driven drug discovery will facilitate precision medicine initiatives, optimizing patient outcomes and market share.
4. Embracing Digital Transformation
Implementing digital health solutions, remote clinical trial models, and AI-driven R&D processes will streamline operations, reduce costs, and accelerate drug development timelines. Digital marketing and patient engagement platforms can improve market penetration and adherence.
5. Entering Emerging Markets
Growing healthcare infrastructure in Asia-Pacific, Latin America, and Africa presents an avenue for revenue growth. Tailoring product offerings and pricing strategies to local needs will enhance market share and support global health objectives.
Challenges and Risks
- Regulatory and Pricing Pressures: Governments worldwide intensify pricing negotiations, potentially constraining margins, particularly for high-cost therapies like Keytruda.
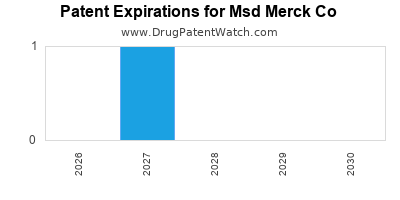
- Patent Expirations: Loss of exclusivity for key products may lead to revenue decline unless replacement therapies are successfully launched.
- Intense Competition: Pharma giants like Roche, Bristol-Myers Squibb, and Novartis actively challenge Merck’s market share through innovation and strategic acquisitions.
- Global Health Dynamics: Evolving pandemic threats and healthcare reforms require agility and resilient supply chains.
Conclusion
MSD Merck’s market dominance stems from its leadership in immunotherapy, vaccines, and R&D capabilities. Strategic focus on innovation, digital transformation, and expanding into emerging markets will underpin its growth trajectory amid mounting competitive pressures. Its ability to adapt swiftly to emerging scientific trends and healthcare demands will determine its sustainability as a leading innovator in the global pharmaceutical landscape.
Key Takeaways
- Merck commands a strong position in oncology, vaccines, and immunotherapies, bolstered by blockbuster products like Keytruda and Gardasil.
- Heavy investment in R&D and strategic collaborations are vital for maintaining innovation leadership and pipeline vitality.
- The company’s expansion into mRNA technology and personalized medicine presents significant growth opportunities.
- Navigating regulatory and pricing environments requires strategic agility to protect margins and sustain profitability.
- Emphasis on emerging market penetration and digital health initiatives will be crucial for long-term growth.
FAQs
1. What is Merck's primary revenue driver?
Keytruda, the immunotherapy drug, remains Merck’s primary revenue driver, accounting for over 30% of its annual sales and celebrating continuous growth through new indications.
2. How is Merck positioning itself in mRNA vaccine development?
Through strategic partnerships with Moderna and investments in mRNA research, Merck aims to innovate beyond COVID-19 vaccines into personalized vaccines for diseases like cancer.
3. How does Merck differentiate itself from competitors like Roche and Bristol-Myers Squibb?
Merck’s differentiation lies in its broad oncology portfolio with Keytruda, a robust vaccine lineup, and its focus on integrating digital health and precision medicine into its pipeline.
4. What are the main challenges facing Merck in the upcoming decade?
Patent expirations, regulatory pricing pressures, fierce competition, and the need for rapid innovation pose significant challenges requiring strategic agility.
5. What growth opportunities does Merck see in emerging markets?
Expanding healthcare infrastructure, increasing vaccination rates, and tailored product strategies offer substantial revenue growth potential in Asia-Pacific, Latin America, and Africa.
Sources
[1] IMS Health, Merck financial reports, 2022.
[2] Merck Annual Report, 2022.
[3] GlobalData, “Pharmaceutical Market Analysis,” 2023.
[4] Statista, “Top Pharmaceutical Companies by Revenue,” 2023.
[5] ClinicalTrials.gov, “Merck R&D Pipeline,” 2023.