TACROLIMUS Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Tacrolimus, and what generic alternatives are available?
Tacrolimus is a drug marketed by Chengdu, Accord Hlthcare, Ajenat Pharms, Alkem Labs Ltd, Biocon Pharma, Concord Biotech Ltd, Dr Reddys Labs Ltd, Glenmark Pharms Ltd, Hangzhou Zhongmei, Heritage Pharma Avet, Mylan, Panacea, Sandoz, Strides Pharma, Hospira, Nexus, Encube, and Fougera Pharms Inc. and is included in twenty NDAs.
The generic ingredient in TACROLIMUS is tacrolimus. There are twenty drug master file entries for this compound. Thirty-four suppliers are listed for this compound. Additional details are available on the tacrolimus profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Tacrolimus
A generic version of TACROLIMUS was approved as tacrolimus by SANDOZ on August 10th, 2009.
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for TACROLIMUS?
- What are the global sales for TACROLIMUS?
- What is Average Wholesale Price for TACROLIMUS?
Summary for TACROLIMUS
| US Patents: | 0 |
| Applicants: | 18 |
| NDAs: | 20 |
| Finished Product Suppliers / Packagers: | 31 |
| Raw Ingredient (Bulk) Api Vendors: | 65 |
| Clinical Trials: | 1,384 |
| Drug Prices: | Drug price information for TACROLIMUS |
| Drug Sales Revenues: | Drug sales revenues for TACROLIMUS |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for TACROLIMUS |
| What excipients (inactive ingredients) are in TACROLIMUS? | TACROLIMUS excipients list |
| DailyMed Link: | TACROLIMUS at DailyMed |
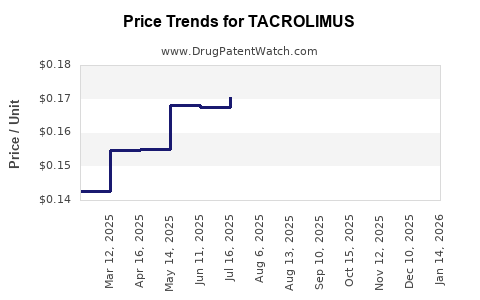
See drug prices for TACROLIMUS
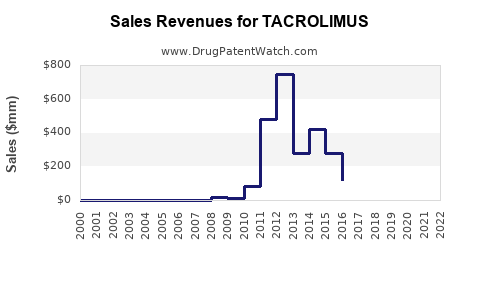
Recent Clinical Trials for TACROLIMUS
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Shalamar Institute of Health Sciences | PHASE3 |
| Sung Won Choi | PHASE2 |
| Milton S. Hershey Medical Center | PHASE2 |
Pharmacology for TACROLIMUS
| Drug Class | Calcineurin Inhibitor Immunosuppressant |
| Mechanism of Action | Calcineurin Inhibitors |
Medical Subject Heading (MeSH) Categories for TACROLIMUS
Anatomical Therapeutic Chemical (ATC) Classes for TACROLIMUS
Paragraph IV (Patent) Challenges for TACROLIMUS
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| ENVARSUS XR | Extended-release Tablets | tacrolimus | 0.75 mg, 1 mg and 4 mg | 206406 | 1 | 2022-03-31 |
| ASTAGRAF XL | Extended-release Capsules | tacrolimus | 0.5 mg, 1 mg, and 5 mg | 204096 | 1 | 2013-09-24 |
| PROTOPIC | Ointment | tacrolimus | 0.03% | 050777 | 1 | 2010-11-22 |
| PROTOPIC | Ointment | tacrolimus | 0.10% | 050777 | 1 | 2010-09-09 |
US Patents and Regulatory Information for TACROLIMUS
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hospira | TACROLIMUS | tacrolimus | INJECTABLE;INJECTION | 203900-001 | Aug 25, 2017 | DISCN | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Panacea | TACROLIMUS | tacrolimus | CAPSULE;ORAL | 090802-001 | Sep 28, 2012 | AB | RX | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Glenmark Pharms Ltd | TACROLIMUS | tacrolimus | OINTMENT;TOPICAL | 210393-001 | Apr 16, 2018 | AB | RX | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Encube | TACROLIMUS | tacrolimus | OINTMENT;TOPICAL | 212387-001 | Oct 10, 2023 | BX | RX | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Heritage Pharma Avet | TACROLIMUS | tacrolimus | CAPSULE;ORAL | 090402-001 | Jul 1, 2010 | DISCN | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
EU/EMA Drug Approvals for TACROLIMUS
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Astellas Pharma Europe B.V. | Modigraf | tacrolimus | EMEA/H/C/000954Prophylaxis of transplant rejection in adult and paediatric, kidney, liver or heart allograft recipients.Treatment of allograft rejection resistant to treatment with other immunosuppressive medicinal products in adult and paediatric patients. | Authorised | no | no | no | 2009-05-15 | |
| Astellas Pharma Europe BV | Advagraf | tacrolimus | EMEA/H/C/000712Prophylaxis of transplant rejection in adult kidney or liver allograft recipients.Treatment of allograft rejection resistant to treatment with other immunosuppressive medicinal products in adult patients. | Authorised | no | no | no | 2007-04-23 | |
| Chiesi Farmaceutici S.p.A. | Envarsus | tacrolimus | EMEA/H/C/002655Prophylaxis of transplant rejection in adult kidney or liver allograft recipients. Treatment of allograft rejection resistant to treatment with other immunosuppressive medicinal products in adult patients. | Authorised | no | no | no | 2014-07-18 | |
| LEO Pharma A/S | Protopic | tacrolimus | EMEA/H/C/000374Flare treatmentAdults and adolescents (16 years of age and above)Treatment of moderate to severe atopic dermatitis in adults who are not adequately responsive to or are intolerant of conventional therapies such as topical corticosteroids.Children (two years of age and above)Treatment of moderate to severe atopic dermatitis in children (two years of age and above) who failed to respond adequately to conventional therapies such as topical corticosteroids.Maintenance treatmentMaintenance treatment of moderate to severe atopic dermatitis for the prevention of flares and the prolongation of flare-free intervals in patients experiencing a high frequency of disease exacerbations (i.e. occurring four or more times per year) who have had an initial response to a maximum of six weeks treatment of twice daily tacrolimus ointment (lesions cleared, almost cleared or mildly affected). | Authorised | no | no | no | 2002-02-27 | |
| Teva B.V. | Tacforius | tacrolimus | EMEA/H/C/004435Prophylaxis of transplant rejection in adult kidney or liver allograft recipients.Treatment of allograft rejection resistant to treatment with other immunosuppressive medicinal products in adult patients. | Authorised | yes | no | no | 2017-12-08 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
Market Dynamics and Financial Trajectory for the Pharmaceutical Drug: Tacrolimus
More… ↓
