Last updated: July 27, 2025
Introduction
Rufinamide is an antiepileptic drug (AED) primarily indicated for Lennox-Gastaut syndrome (LGS), a severe form of epilepsy characterized by multiple seizure types and resistance to conventional treatments. Approved by the U.S. Food and Drug Administration (FDA) in 2009 under the brand name Banzel (manufactured by Eisai Co., Ltd.), rufinamide has contributed significantly to the therapeutic landscape for refractory epilepsies. This report analyzes current market dynamics and projects the financial trajectory for rufinamide over the coming years, considering factors such as clinical demand, regulatory landscape, competitive environment, and socio-economic drivers.
Market Overview and Therapeutic Indications
The primary market for rufinamide centers on Lennox-Gastaut syndrome, a rare but severe epilepsy form affecting approximately 1-10 per 10,000 individuals globally. The rarity of LGS positions rufinamide as part of an orphan drug category, influencing pricing, market penetration, and regulatory support.
Beyond LGS, emerging evidence and off-label use suggest potential expansion into other refractory epilepsies, although these uses remain limited pending further clinical validation. The drug's mechanism, a modulation of voltage-gated sodium channels, offers a targeted approach for seizure control, aligning with personalized medicine trends.
Market Dynamics
1. Prevalence and Unmet Needs
The niche nature of LGS sustains a steady, albeit limited, demand for rufinamide. Despite advances in epilepsy management, these patients often experience drug-resistant seizures, creating consistent demand for effective treatments like rufinamide.
Unmet needs include improved seizure control, reduced side effects, and more cost-effective therapies. Rufinamide addresses these needs but faces competition from newer drugs with better tolerability or broader indications.
2. Regulatory and Patent Landscape
Initially approved under orphan drug designations, rufinamide benefits from market exclusivity periods that typically extend for 7 years in the US. However, patent expirations or the absence of patent protections in some regions expose the drug to generic competition, impacting pricing and market share.
Eisai has maintained regulatory exclusivity in key markets; however, the patent landscape varies in emerging markets, where generic versions may erode revenue streams.
3. Competitive Environment
Secondary competitors include other AEDs such as cannabidiol (Epidiolex), stiripentol, topiramate, and clobazam, all indicated for different aspects of epilepsy management. Apixaban and other new agents lack direct competition but pose substitution threats in wider epilepsy treatment.
Importantly, the evolving pipeline and ongoing clinical trials exploring combination therapies or novel mechanisms could influence rufinamide's market share.
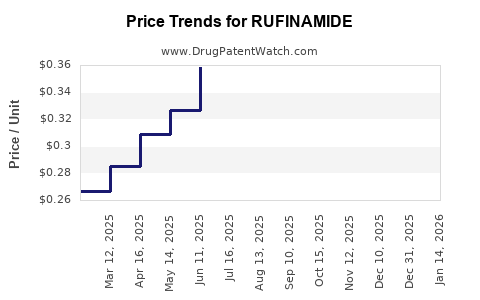
4. Pricing and Reimbursement Policies
Originator pricing remains premium, justified by clinical efficacy, safety profile, and orphan drug status. However, reimbursement constraints and cost-containment measures, especially in成熟 markets like Europe and North America, pressure profit margins.
In emerging economies, price sensitivity and regulatory hurdles limit access, restricting potential revenue growth.
5. Clinical and Research Developments
Recent clinical trials have explored rufinamide's efficacy in other epileptic syndromes and off-label indications. Positive trial outcomes could expand the market, although regulatory approval processes could delay commercialization.
Furthermore, real-world evidence and post-marketing surveillance support the drug’s safety profile, bolstering its position in treatment guidelines.
Financial Trajectory Analysis
1. Revenue Forecasts
Eisai’s financial disclosures indicate that rufinamide contributed approximately $100-150 million annually to the company’s epilepsy portfolio, with growth margins stabilized in recent years. Going forward, revenue projections are based on:
- Market penetration levels: Stabilization in developed markets.
- Patent expiry impacts: Potential declines in the U.S. and Europe from 2026 onward.
- Generic competition: Entry could reduce pricing and overall revenue by 30-50%, depending on market penetration.
2. Unit Volume and Pricing Trends
The volume of prescriptions for rufinamide remains steady within its niche but may plateau with the saturation of eligible patients. Prices, however, may decline due to increased competition or regional pricing negotiations.
New formulations, such as liquid or alternative delivery forms, could sustain or boost unit sales.
3. Cost Considerations
Manufacturing costs are relatively stable but may rise with supply chain disruptions or increased regulatory compliance expenses. Marketing and physician education investments influence sales but are balanced against margins.
4. Strategic Growth Initiatives
Eisai's pipeline development, including combination therapies and potential new indications, offers avenues for revenue diversification. Expanding into emerging markets through licensing or partnerships could realize incremental growth but may dilute profit margins.
5. Long-term Financial Outlook
Assuming stable demand in core markets, the revenue trajectory for rufinamide is expected to plateau over the next 3-5 years. From approximately $120 million currently, projections suggest potential declines post-2026 due to patent cliffs unless new indications or formulations emerge.
However, early-stage clinical trials and expanding access in emerging regions might offset declines, maintaining revenues in the $80-100 million range beyond 2025.
Strategic Market Opportunities
- Indication Expansion: Investigator-led studies into other epilepsies could generate secondary demand.
- Formulation Innovation: Developing extended-release or pediatric-friendly formulations to enhance compliance.
- Geographical Penetration: Accelerating approvals in Latin America, Asia, and Africa to broaden the market base.
- Regulatory Pathways: Leveraging orphan drug exclusivities and Fast Track designations to expedite commercialization of new formulations or indications.
Risks and Challenges
- Patent expiration and generic competition threaten revenue streams.
- Emergence of superior therapies or combination regimens could diminish rufinamide's market share.
- Regulatory restrictions or pricing pressures in key markets may constrict profit margins.
- Supply chain vulnerabilities could impair manufacturing, impacting availability and sales.
Conclusion
Rufinamide remains a niche yet vital treatment for Lennox-Gastaut syndrome, with a stable market driven by clinical need and regulatory protections. Its financial trajectory depends on patent and regulatory developments, competitive dynamics, and strategic initiatives targeting expansion and innovation. While near-term revenues are expected to stabilize, long-term growth will rely on indication expansion, market access strategies, and innovation in formulation.
Key Takeaways
- Steady Demand in Niche Market: Rufinamide’s primary application in LGS sustains consistent demand; however, growth potential is limited outside its approved indication.
- Patent and Competition Risks: Upcoming patent expirations and the entry of generics are significant factors influencing pricing and revenue decline post-2026.
- Regulatory and Market Expansion Opportunities: Expanding into emerging markets and exploring additional indications could offset revenue erosion.
- Pipeline and Formulation Innovation: Developing new formulations and seeking approval for off-label uses could extend the product lifecycle.
- Strategic Focus: Success hinges on leveraging regulatory exclusivities, cost-effective manufacturing, and targeted clinical research.
FAQs
1. When is rufinamide expected to face generic competition?
Patent protection in the US and other key markets extends until approximately 2026-2028, beyond which generics are expected to enter, likely reducing prices and market share.
2. Can rufinamide’s indications expand beyond Lennox-Gastaut syndrome?
While current approval is limited to LGS, ongoing clinical trials and research may support expansion into other epileptic syndromes, subject to regulatory approval.
3. How does the orphan drug status influence rufinamide’s market and pricing?
Orphan designation grants market exclusivity, incentivizing high pricing and market stability. However, expiration of exclusivity exposes it to competition, impacting revenues.
4. What emerging therapies could threaten rufinamide’s position?
New AEDs such as cannabidiol (Epidiolex), brivaracetam, and gene therapies targeting epilepsy may offer alternatives, challenging rufinamide’s dominance in its niche.
5. Is there potential for rufinamide in drug combination therapies?
Research suggests combining rufinamide with other AEDs might improve efficacy; however, clinical validation and regulatory approval are necessary before mainstream adoption.
Sources:
[1] FDA Approvals and Drug Labels
[2] Eisai Co., Ltd. Financial Reports
[3] ClinicalTrials.gov
[4] Industry Market Research Reports
[5] European Medicines Agency (EMA) Data