LITHIUM CARBONATE Drug Patent Profile
✉ Email this page to a colleague
When do Lithium Carbonate patents expire, and when can generic versions of Lithium Carbonate launch?
Lithium Carbonate is a drug marketed by Able, Alembic Ltd, Apotex Inc, Glenmark Generics, Hetero Labs Ltd Iii, Hikma, Mylan, Usl Pharma, Watson Labs, Alembic, Glenmark Pharms Inc, Heritage Pharma, Hikma Intl Pharms, Mylan Pharms Inc, Unique, Pfizer, and Sun Pharm Inds Inc. and is included in twenty-nine NDAs.
The generic ingredient in LITHIUM CARBONATE is lithium carbonate. There are fifteen drug master file entries for this compound. Twenty-two suppliers are listed for this compound. Additional details are available on the lithium carbonate profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Lithium Carbonate
A generic version of LITHIUM CARBONATE was approved as lithium carbonate by HIKMA on January 29th, 1982.
Summary for LITHIUM CARBONATE
| US Patents: | 0 |
| Applicants: | 17 |
| NDAs: | 29 |
| Finished Product Suppliers / Packagers: | 21 |
| Raw Ingredient (Bulk) Api Vendors: | 133 |
| Clinical Trials: | 98 |
| Patent Applications: | 101 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for LITHIUM CARBONATE |
| What excipients (inactive ingredients) are in LITHIUM CARBONATE? | LITHIUM CARBONATE excipients list |
| DailyMed Link: | LITHIUM CARBONATE at DailyMed |
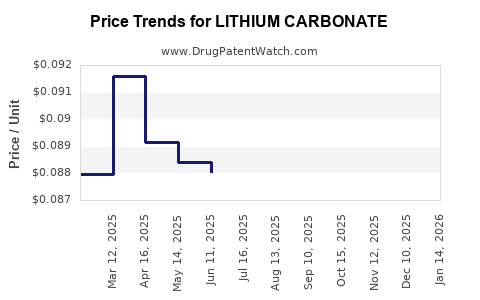
See drug prices for LITHIUM CARBONATE
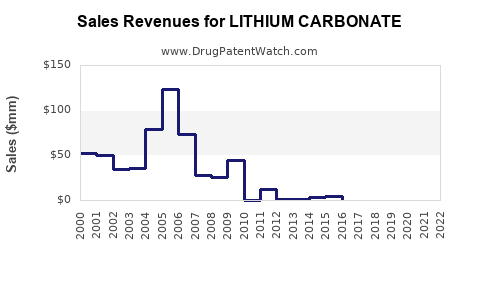
Recent Clinical Trials for LITHIUM CARBONATE
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Second Xiangya Hospital of Central South University | Phase 1/Phase 2 |
| First Affiliated Hospital of Chongqing Medical University | Phase 1/Phase 2 |
| Academisch Medisch Centrum - Universiteit van Amsterdam (AMC-UvA) | Phase 2 |
Pharmacology for LITHIUM CARBONATE
| Drug Class | Mood Stabilizer |
Medical Subject Heading (MeSH) Categories for LITHIUM CARBONATE
Anatomical Therapeutic Chemical (ATC) Classes for LITHIUM CARBONATE
US Patents and Regulatory Information for LITHIUM CARBONATE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hikma Intl Pharms | LITHIUM CARBONATE | lithium carbonate | TABLET, EXTENDED RELEASE;ORAL | 076490-001 | Jun 17, 2003 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Heritage Pharma | LITHIUM CARBONATE | lithium carbonate | TABLET, EXTENDED RELEASE;ORAL | 205532-001 | Sep 29, 2016 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Mylan Pharms Inc | LITHIUM CARBONATE | lithium carbonate | TABLET, EXTENDED RELEASE;ORAL | 202288-001 | Jun 29, 2012 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Heritage Pharma | LITHIUM CARBONATE | lithium carbonate | TABLET, EXTENDED RELEASE;ORAL | 076366-001 | Aug 21, 2003 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Able | LITHIUM CARBONATE | lithium carbonate | CAPSULE;ORAL | 076823-001 | Jun 29, 2004 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Mylan | LITHIUM CARBONATE | lithium carbonate | CAPSULE;ORAL | 076243-002 | Feb 24, 2003 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |


