Last updated: July 27, 2025
Introduction
Lacosamide (brand name Vimpat), a novel antiepileptic drug (AED), has established itself within the niche of neurological pharmacotherapy, primarily targeting focal seizures. Approved by the U.S. Food and Drug Administration (FDA) in 2008, lacosamide's unique mechanism—enhancing slow inactivation of voltage-gated sodium channels—differentiates it from older AEDs and positions it as a significant player in epilepsy management. Understanding lacosamide's evolving market dynamics and financial trajectory offers valuable insights for stakeholders, including pharmaceutical companies, investors, and healthcare policy planners.
Market Landscape and Therapeutic Positioning
Prevalence of Epilepsy and Market Demand
Epilepsy affects approximately 50 million people worldwide, with focal seizures comprising about 60% of adult cases (WHO, 2019). As a result, the global antiepileptic drug market is projected to reach USD 5.5 billion by 2027, growing at a compound annual growth rate (CAGR) of around 4%, driven by increasing prevalence and broader adoption of innovative therapies (Fortune Business Insights, 2022).
Lacosamide’s Clinical Edge
Lacosamide’s pharmacological profile suits patients with partial-onset seizures, either as adjunct or monotherapy. Its favorable tolerability profile, characterized by minimal cognitive impairment and manageable side effects, has facilitated its acceptance among neurologists. Regulatory approvals across multiple markets, including the U.S., Europe, and Japan, have broadened geographic reach. The drug’s positioning as a second-generation AED highlights its incremental value over traditional agents, emphasizing efficacy and improved safety.
Competitive Considerations
Lacosamide faces competition from a spectrum of AEDs, such as levetiracetam, lamotrigine, and carbamazepine. Its incremental differentiation hinges on its efficacy in refractory cases, compatibility with other medications, and reduced cognitive side effects. However, the advent of newer agents and the emergence of generic versions influence its market share dynamics.
Market Dynamics Influencing Lacosamide
Regulatory and Patent Trajectories
Initially protected by patents extending into the 2020s, lacosamide’s exclusivity has begun to diminish with patent expirations and the entry of generic competitors. The consolidation of market share post-patent expiry poses both challenges and opportunities—while generic pricing pressures compress revenues, increased accessibility can expand overall utilization.
Pricing and Reimbursement Policies
Pricing strategies and reimbursement frameworks across different regions substantially impact lacosamide sales. In developed markets like the U.S. and Europe, reimbursement coverage, formulary placements, and prescribing guidelines influence market penetration. Cost-effectiveness analyses favoring lacosamide, especially in refractory epilepsy, have facilitated favorable formulary placements.
Distribution Channels and Prescriber Dynamics
Neurologists and epileptologists are primary prescribers, with specialist oversight impacting uptake. Distribution channels—hospital pharmacies, retail pharmacies, and specialty clinics—differ by region. Education regarding its benefits and safety profile continues to be critical in expanding prescriber confidence.
Emerging Disease and Market Trends
The trend towards personalized medicine and biomarker-guided therapies could modify lacosamide's role, emphasizing its use in refractory cases and specific patient subsets. Additionally, the rising trend of polytherapy, where lacosamide's adjunct use is favored, sustains its demand.
Financial Trajectory and Revenue Forecasts
Historical Revenue Performance
Lacosamide's revenues grew steadily post-launch, peaking around USD 600 million globally in 2019. Precise figures fluctuate based on regional sales, patent status, and generic competition, with North America representing approximately 50-60% of sales (EvaluatePharma, 2021).
Impact of Patent Expiry and Generics
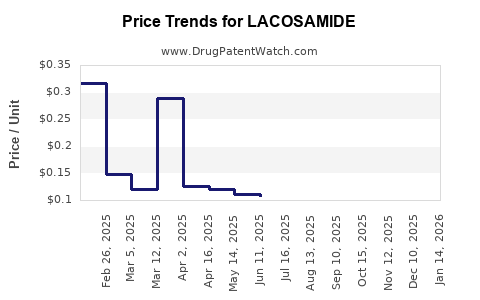
Patent expiration in major markets is anticipated between 2023-2025. Generic entrants are expected to reduce per-unit prices sharply—by up to 70%—while volumes could increase due to enhanced affordability and accessibility. This transition will shape revenue trajectories, potentially causing a decline in branded sales but an overall expansion in market penetration.
Forecasted Trends (2023-2030)
Analysts project that lacosamide’s global sales may experience a dip through 2025 due to generic competition. However, growth could stabilize or rebound as utilization shifts towards broader indications, including investigational uses in neuropathic pain and traumatic brain injury (Phase II trials). The total market for lacosamide is forecasted to decline modestly at a CAGR of 2-3% through the late 2020s, stabilizing at approximately USD 1.2 billion in sales by 2030.
Emerging Opportunities and Risks
-
Expansion into Adjunct Indications: Clinical trials investigating lacosamide for neuropathic pain, mood disorders, and special populations could unlock new revenue streams, subject to regulatory approval.
-
Generic Competition: The onset of biosimilars and generics remains the primary risk to revenue longevity.
-
Pricing and Reimbursement Policies: Stricter cost containment measures threaten higher-margin sales, especially in publicly funded healthcare systems.
-
Market Penetration in Emerging Economies: Increasing prevalence of epilepsy and expanding healthcare infrastructure in Asia-Pacific, Latin America, and Africa offer long-term growth opportunities.
Global Market Penetration and Key Region Analysis
North America
The U.S. remains the largest single market attributable to high epilepsy prevalence, advanced healthcare infrastructure, and robust reimbursement pathways. Despite impending patent expiration, strong brand loyalty and existing formulary placements sustain revenues. Strategic marketing and clinical evidence continue to underpin market dominance.
Europe
Regulatory approval through the European Medicines Agency (EMA) facilitated a mature market, with sales bolstered by reimbursements and neurologist endorsements. Price negotiations and health technology assessments (HTAs) influence market share.
Asia-Pacific
Rapid growth potential exists due to rising epilepsy prevalence, increasing healthcare expenditure, and expanding neurologist density. Nevertheless, regulatory complexities and price sensitivities require tailored market strategies.
Emerging Markets
Market entry and expansion in Latin America, Africa, and the Middle East are capitalized on by licensing agreements and local partnerships. Affordability remains a key barrier, mitigated by the introduction of generics.
Concluding Insights
Lacosamide exemplifies a therapeutically innovative yet increasingly commoditized antiseizure agent. Its market dynamics are shaped significantly by patent expirations, evolving clinical guidelines, and healthcare policies prioritizing cost-effectiveness. Financially, the drug’s trajectory will likely follow a decline in branded revenue post-patent expiry, complemented by volume-driven growth in emerging markets. Companies focusing on lifecycle management, such as expanding indications and geographic footprint, will be best positioned to leverage lacosamide’s enduring therapeutic niche.
Key Takeaways
- Patent expiration and generic competition will exert downward pressure on lacosamide’s revenues in mature markets starting in 2023–2025.
- Market expansion into emerging economies offers long-term growth opportunities, driven by rising epilepsy prevalence and healthcare infrastructure development.
- Clinical research into additional indications could mitigate revenue declines and unlock new revenue streams.
- Pricing and reimbursement strategies will be pivotal—aligning with healthcare policies ensures sustained market presence.
- Strategic lifecycle management, including differentiation and geographic expansion, can extend lacosamide’s financial viability.
FAQs
1. How will patent expiration affect lacosamide’s market share?
Patent expiration will enable generic competitors to enter the market, leading to significant price reductions and potential erosion of branded revenues, although increased volume and broader access can partially offset declines.
2. What are the primary factors influencing lacosamide’s adoption in developing countries?
Cost sensitivity, healthcare infrastructure, regulatory approval processes, and availability of generics are key factors. Local partnerships and pricing strategies are essential for market penetration.
3. Are there emerging indications that could extend lacosamide’s market life?
Yes, ongoing clinical trials investigate its use in neuropathic pain, mood disorders, and neuroprotection, which could diversify its application beyond epilepsy.
4. How significant is the competition from other second-generation AEDs?
Competition is intense, with drugs like levetiracetam and lacosamide vying for refractory epilepsy niches. Differentiation based on tolerability and efficacy shapes competitive positioning.
5. What strategic priorities should pharmaceutical companies focus on to maximize lacosamide’s financial trajectory?
Lifecycle management through indication expansion, optimizing pricing and reimbursement strategies, strengthening presence in emerging markets, and clinical evidence generation are critical.
References
- World Health Organization. (2019). Epilepsy Fact Sheet.
- Fortune Business Insights. (2022). Global Antiepileptic Drugs Market Size, Share & Industry Analysis.
- EvaluatePharma. (2021). Annual Review of Global Epilepsy Drug Sales and Patent Status.