APIXABAN Drug Patent Profile
✉ Email this page to a colleague
When do Apixaban patents expire, and when can generic versions of Apixaban launch?
Apixaban is a drug marketed by Accord Hlthcare, Apotex, Aurobindo Pharma Ltd, Bionpharma, Breckenridge, Hetero Labs Ltd V, Impax, Macleods Pharms Ltd, Micro Labs, Mylan, Regcon Holdings, Torrent, and Zydus Pharms. and is included in thirteen NDAs.
The generic ingredient in APIXABAN is apixaban. There are thirty drug master file entries for this compound. Eleven suppliers are listed for this compound. Additional details are available on the apixaban profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Apixaban
A generic version of APIXABAN was approved as apixaban by ACCORD HLTHCARE on July 28th, 2020.
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for APIXABAN?
- What are the global sales for APIXABAN?
- What is Average Wholesale Price for APIXABAN?
Summary for APIXABAN
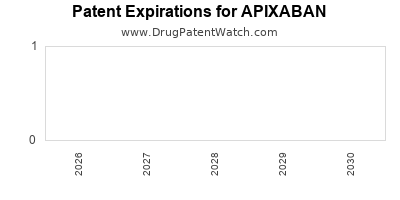
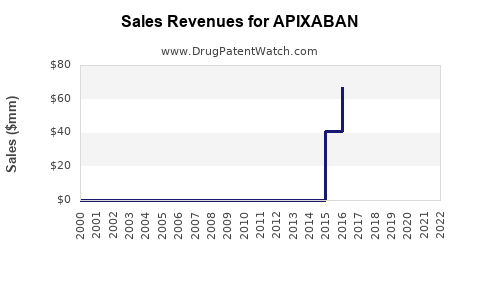
Recent Clinical Trials for APIXABAN
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| University of Birmingham | PHASE3 |
| University of Melbourne | PHASE4 |
| Emily McDonald | PHASE4 |
Pharmacology for APIXABAN
| Drug Class | Factor Xa Inhibitor |
| Mechanism of Action | Factor Xa Inhibitors |
Anatomical Therapeutic Chemical (ATC) Classes for APIXABAN
US Patents and Regulatory Information for APIXABAN
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Accord Hlthcare | APIXABAN | apixaban | TABLET;ORAL | 210180-001 | Jul 28, 2020 | AB | RX | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Micro Labs | APIXABAN | apixaban | TABLET;ORAL | 210013-002 | Dec 23, 2019 | DISCN | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Torrent | APIXABAN | apixaban | TABLET;ORAL | 210156-002 | Dec 17, 2024 | DISCN | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Bionpharma | APIXABAN | apixaban | TABLET;ORAL | 210152-001 | Apr 8, 2020 | DISCN | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
EU/EMA Drug Approvals for APIXABAN
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Bristol-Myers Squibb / Pfizer EEIG | Eliquis | apixaban | EMEA/H/C/002148For Eliquis 2.5 mg film-coated tablets:Prevention of venous thromboembolic events (VTE) in adult patients who have undergone elective hip or knee replacement surgery.Prevention of stroke and systemic embolism in adult patients with non-valvular atrial fibrillation (NVAF), with one or more risk factors, such as prior stroke or transient ischaemic attack (TIA); age ≥ 75 years; hypertension; diabetes mellitus; symptomatic heart failure (NYHA Class ≥ II).Treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE), and prevention of recurrent DVT and PE in adults (see section 4.4 for haemodynamically unstable PE patients).For Eliquis 5 mg film-coated tablets:Prevention of stroke and systemic embolism in adult patients with non-valvular atrial fibrillation (NVAF), with one or more risk factors, such as prior stroke or transient ischaemic attack (TIA); age≥ 75 years; hypertension; diabetes mellitus; symptomatic heart failure (NYHA Class ≥ II).Treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE), and prevention of recurrent DVT and PE in adults (see section 4.4 for haemodynamically unstable PE patients). | Authorised | no | no | no | 2011-05-18 | |
| Accord Healthcare S.L.U. | Apixaban Accord | apixaban | EMEA/H/C/005358Prevention of venous thromboembolic events (VTE) in adult patients who have undergone elective hip or knee replacement surgery.Prevention of stroke and systemic embolism in adult patients with non-valvular atrial fibrillation (NVAF), with one or more risk factors, such as prior stroke or transient ischaemic attack (TIA); age ≥ 75 years; hypertension; diabetes mellitus; symptomatic heart failure (NYHA Class ≥ II).Treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE), and prevention of recurrent DVT and PE in adults (see section 4.4 for haemodynamically unstable PE patients).Prevention of stroke and systemic embolism in adult patients with non-valvular atrial fibrillation (NVAF), with one or more risk factors, such as prior stroke or transient ischaemic attack (TIA); age≥ 75 years; hypertension; diabetes mellitus; symptomatic heart failure (NYHA Class ≥ II).Treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE), and prevention of recurrent DVT and PE in adults (see section 4.4 for haemodynamically unstable PE patients). | Authorised | yes | no | no | 2020-07-23 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
Market Dynamics and Financial Trajectory for Apixaban
More… ↓
