Roflumilast - Generic Drug Details
✉ Email this page to a colleague
What are the generic drug sources for roflumilast and what is the scope of patent protection?
Roflumilast
is the generic ingredient in three branded drugs marketed by Arcutis, Astrazeneca, Alkem Labs Ltd, Aurobindo Pharma Ltd, Breckenridge, Hetero Labs Ltd Iii, Micro Labs, MSN, Mylan, Prinston Inc, Strides Pharma, Torrent, and Zydus Pharms, and is included in fourteen NDAs. There are sixteen patents protecting this compound and one Paragraph IV challenge. Additional information is available in the individual branded drug profile pages.Roflumilast has eighty-five patent family members in twenty-nine countries.
There are ten drug master file entries for roflumilast. Fourteen suppliers are listed for this compound.
Summary for roflumilast
| International Patents: | 85 |
| US Patents: | 16 |
| Tradenames: | 3 |
| Applicants: | 13 |
| NDAs: | 14 |
| Drug Master File Entries: | 10 |
| Finished Product Suppliers / Packagers: | 14 |
| Raw Ingredient (Bulk) Api Vendors: | 136 |
| Clinical Trials: | 99 |
| Patent Applications: | 7,299 |
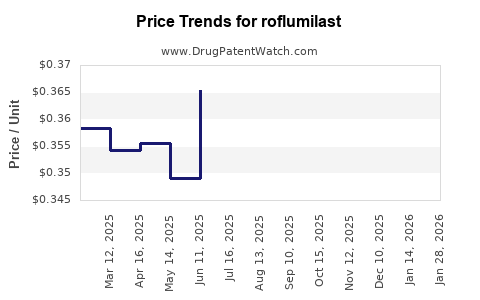
| Drug Prices: | Drug price trends for roflumilast |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for roflumilast |
| What excipients (inactive ingredients) are in roflumilast? | roflumilast excipients list |
| DailyMed Link: | roflumilast at DailyMed |
Recent Clinical Trials for roflumilast
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Mansoura University | Phase 1/Phase 2 |
| The Novo Nordic Foundation | Phase 4 |
| Jacob Pontoppidan Thyssen | Phase 4 |
Pharmacology for roflumilast
| Drug Class | Phosphodiesterase 4 Inhibitor |
| Mechanism of Action | Phosphodiesterase 4 Inhibitors |
Anatomical Therapeutic Chemical (ATC) Classes for roflumilast
Paragraph IV (Patent) Challenges for ROFLUMILAST
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| ZORYVE | Cream | roflumilast | 0.3% | 215985 | 1 | 2023-12-27 |
| DALIRESP | Tablets | roflumilast | 250 mcg | 022522 | 1 | 2019-01-25 |
| DALIRESP | Tablets | roflumilast | 500 mcg | 022522 | 7 | 2015-03-02 |
US Patents and Regulatory Information for roflumilast
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arcutis | ZORYVE | roflumilast | CREAM;TOPICAL | 215985-002 | Jul 9, 2024 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Zydus Pharms | ROFLUMILAST | roflumilast | TABLET;ORAL | 208303-002 | Apr 18, 2023 | AB | RX | No | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| Arcutis | ZORYVE | roflumilast | CREAM;TOPICAL | 215985-001 | Jul 29, 2022 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Arcutis | ZORYVE | roflumilast | FOAM;TOPICAL | 217242-001 | Dec 15, 2023 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Aurobindo Pharma Ltd | ROFLUMILAST | roflumilast | TABLET;ORAL | 213298-001 | Apr 17, 2023 | AB | RX | No | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| Arcutis | ZORYVE | roflumilast | CREAM;TOPICAL | 215985-002 | Jul 9, 2024 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Arcutis | ZORYVE | roflumilast | FOAM;TOPICAL | 217242-001 | Dec 15, 2023 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for roflumilast
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Astrazeneca | DALIRESP | roflumilast | TABLET;ORAL | 022522-001 | Feb 28, 2011 | ⤷ Sign Up | ⤷ Sign Up |
| Astrazeneca | DALIRESP | roflumilast | TABLET;ORAL | 022522-001 | Feb 28, 2011 | ⤷ Sign Up | ⤷ Sign Up |
| Astrazeneca | DALIRESP | roflumilast | TABLET;ORAL | 022522-002 | Jan 23, 2018 | ⤷ Sign Up | ⤷ Sign Up |
| Astrazeneca | DALIRESP | roflumilast | TABLET;ORAL | 022522-001 | Feb 28, 2011 | ⤷ Sign Up | ⤷ Sign Up |
| Astrazeneca | DALIRESP | roflumilast | TABLET;ORAL | 022522-001 | Feb 28, 2011 | ⤷ Sign Up | ⤷ Sign Up |
| Astrazeneca | DALIRESP | roflumilast | TABLET;ORAL | 022522-002 | Jan 23, 2018 | ⤷ Sign Up | ⤷ Sign Up |
| Astrazeneca | DALIRESP | roflumilast | TABLET;ORAL | 022522-002 | Jan 23, 2018 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for roflumilast
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| AstraZeneca AB | Daxas | roflumilast | EMEA/H/C/001179 Daxas is indicated for maintenance treatment of severe chronic obstructive pulmonary disease (COPD) (FEV1 post-bronchodilator less than 50% predicted) associated with chronic bronchitis in adult patients with a history of frequent exacerbations as add-on to bronchodilator treatment., |
Authorised | no | no | no | 2010-07-05 | |
| AstraZeneca AB | Libertek | roflumilast | EMEA/H/C/002399 Libertek is indicated for maintenance treatment of severe chronic obstructive pulmonary disease (COPD) (FEV1 post-bronchodilator less than 50% predicted) associated with chronic bronchitis in adult patients with a history of frequent exacerbations as add-on to bronchodilator treatment. |
Withdrawn | no | no | no | 2011-02-28 | |
| AstraZeneca AB | Daliresp | roflumilast | EMEA/H/C/002398 Daliresp is indicated for maintenance treatment of severe chronic obstructive pulmonary disease (COPD) (FEV1 post-bronchodilator less than 50% predicted) associated with chronic bronchitis in adult patients with a history of frequent exacerbations as add-on to bronchodilator treatment. |
Withdrawn | no | no | no | 2011-02-28 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for roflumilast
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| South Korea | 101179012 | ⤷ Sign Up | |
| Japan | 2006519818 | ⤷ Sign Up | |
| China | 108992673 | 罗氟司特晶体生长的抑制 (Inhibition of crystal growth of roflumilast) | ⤷ Sign Up |
| Iceland | 2723 | ⤷ Sign Up | |
| Australia | 2021393513 | TOPICAL ROFLUMILAST FORMULATION HAVING ANTIFUNGAL PROPERTIES | ⤷ Sign Up |
| China | 115551478 | 具有改善递送和血浆半衰期的罗氟司特局部制剂 (Topical formulations of roflumilast with improved delivery and plasma half-life) | ⤷ Sign Up |
| World Intellectual Property Organization (WIPO) | 2004080967 | ⤷ Sign Up | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for roflumilast
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0706513 | SPC/GB10/040 | United Kingdom | ⤷ Sign Up | PRODUCT NAME: ROFLUMILAST, ROFLUMILAST-N-OXIDE AND THE SALTS OF THESE COMPOUNDS.; REGISTERED: UK EU/1/10/636/001 20100705; UK EU/1/10/636/002 20100705; UK EU/1/10/636/003 20100705 |
| 0706513 | 1090034-8 | Sweden | ⤷ Sign Up | PRODUCT NAME: ROFLUMILAST OCH SALTER DAERAV; REG. NO/DATE: EU/1/10/636/001 20100705 |
| 1606261 | PA2010010,C1606261 | Lithuania | ⤷ Sign Up | PRODUCT NAME: ROFLUMILASTUM; REGISTRATION NO/DATE: EU/1/10/636/001-003 20100705 |
| 1606261 | C20100008 00033 | Estonia | ⤷ Sign Up | PRODUCT NAME: DAXAS-ROFLUMILAST; REG NO/DATE: K(2010)4785 05.07.2010 |
| 0706513 | 34/2010 | Austria | ⤷ Sign Up | PRODUCT NAME: ROFLUMILAST UND DIE SALZE DIESER VERBINDUNG; REGISTRATION NO/DATE: EU/1/10/636/001 - EU/1/10/636/003 20100705 |
| 1606261 | PA2010010 | Lithuania | ⤷ Sign Up | PRODUCT NAME: ROFLUMILASTUM; REGISTRATION NO/DATE: EU/1/10/636/001-003 20100705 |
| 1606261 | C 2010 014 | Romania | ⤷ Sign Up | PRODUCT NAME: ROFLUMILAST; NATIONAL AUTHORISATION NUMBER: RO EU/1/10/636/001, RO EU/1/10/636/002, RO EU/1/10/636/003; DATE OF NATIONAL AUTHORISATION: 20100705; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EMEA EU/1/10/636/001, EMEA EU/1/10/636/002, EMEA EU/1/10/636/003; DATE OF FIRST AUTHORISATION IN EEA: 20100705 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.